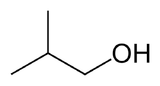
Isobutanol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-methylpropan-1-ol | |
| Other names
Isobutyl alcohol, IBA, 2-methyl-1-propanol, 2-methylpropyl alcohol, Isopropylcarbinol | |
| Identifiers | |
| 78-83-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:46645 |
| ChEMBL | ChEMBL269630 |
| ChemSpider | 6312 |
| ECHA InfoCard | 100.001.044 |
| EC Number | 201-148-0 |
| KEGG | C14710 |
| PubChem | 6560 |
| RTECS number | NP9625000 |
| UNII | 56F9Z98TEM |
| |
| |
| Properties[1] | |
| C4H10O | |
| Molar mass | 74.122 g/mol |
| Appearance | Colorless liquid |
| Odor | sweet, musty[2] |
| Density | 0.802 g/cm3, liquid |
| Melting point | −108 °C (−162 °F; 165 K) |
| Boiling point | 107.89 °C (226.20 °F; 381.04 K) |
| 8.7 mL/100 mL[3] | |
| log P | 0.8 |
| Vapor pressure | 9 mmHg (20°C)[2] |
| Refractive index (nD) |
1.3959 |
| Viscosity | 3.95 cP at 20 °C |
| Hazards[1] | |
| Safety data sheet | ICSC 0113 |
| EU classification (DSD) |
Irritant (Xi) |
| R-phrases | R10 R37/38 R41, R67 |
| S-phrases | (S2) S7/9 S13 S26 S37/39 S46 |
| NFPA 704 | |
| Flash point | 28 °C (82 °F; 301 K) |
| 415 °C (779 °F; 688 K) | |
| Explosive limits | 1.7–10.9% |
| Lethal dose or concentration (LD, LC): | |
| LDLo (lowest published) |
3750 mg/kg (rabbit, oral) 2460 mg/kg (rat, oral)[4] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 100 ppm (300 mg/m3)[2] |
| REL (Recommended) |
TWA 50 ppm (150 mg/m3)[2] |
| IDLH (Immediate danger) |
1600 ppm[2] |
| Related compounds | |
| Related butanols |
n-Butanol sec-Butanol tert-Butanol |
| Related compounds |
Isobutyraldehyde Isobutyric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent. Its isomers, the other butanols, include n-butanol, 2-butanol, and tert-butanol, all of which are important industrially.
Production
Isobutanol is produced by the carbonylation of propylene. Two methods are practiced industrially, hydroformylation is more common and generates a mixture of isobutyraldehydes, which are hydrogenated to the alcohols and then separated. Reppe carbonylation is also practiced.[5]
Biosynthesis of Isobutanol
Higher-chain alcohols have energy densities close to gasoline, are not as volatile or corrosive as ethanol, and do not readily absorb water. Furthermore, branched-chain alcohols, such as isobutanol, have higher-octane numbers, resulting in less knocking in engines. Although produced naturally during the fermentation of saccharides and may also be a byproduct of the decay process of organic matter, Isobutanol or C5 alcohols have never been produced from a renewable source with yields high enough to make them viable as a gasoline substitute before the 2008 Nature article that produced over 20g/L isobutanol from glucose in E.coli.[6]
To modify an organism to produce these compounds usually results in toxicity in the cell. This difficulty was bypassed by leveraging the native metabolic networks in E. coli but altered its intracellular chemistry using genetic engineering to produce these alcohols. Key pathways in E. coli were modified to produce several higher-chain alcohols from glucose, including isobutanol, 1-butanol, 2-methyl-1-butanol, 3-methyl-1-butanol, and 2-phenylethanol. This strategy exploits the E. coli host's highly active amino acid biosynthetic pathway by shifting part of it to alcohol production. It is proposed that these unusual alcohols can be produced as efficiently as the biosynthesis of ethanol.[6]
Escherichia coli
Escherichia coli, or E. coli, is a Gram-negative, rod-shaped bacteria. E. coli is the microorganism most likely to move on to commercial production of isobutanol.[6][7] In its engineered form E. coli produces the highest yields of isobutanol of any microorganism.[6] Methods such as elementary mode analysis have been used to improve the metabolic efficiency of E. coli so that larger quantities of isobutanol may be produced.[8] E. coli is an ideal isobutanol bio-synthesizer for several reasons:
- E. coli is an organism for which several tools of genetic manipulation exist, and it is an organism for which an extensive body of scientific literature exists.[7] This wealth of knowledge allows E. coli to be easily modified by scientists.
- E. coli has the capacity to use lignocellulose (waste plant matter leftover from agriculture) in the synthesis of isobutanol. The use of lignocellulose prevents E. coli from using plant matter meant for human consumption, and prevents any food-fuel price relationship which would occur from the biosynthesis of isobutanol by E. coli.[7]
- Genetic modification has been used to broaden the scope of lignocellulose which can be used by E. coli. This has made E. coli a useful and diverse isobutanol bio-synthesizer.[9]
The primary drawback of E. coli is that it is susceptible to bacteriophages when being grown. This susceptibility could potentially shut down entire bioreactors.[7]
Clostridium
While cellulosic biomass like corn stover and switchgrass is abundant and cheap, it is much more difficult to utilize than corn and sugar cane. This is due in large part because of recalcitrance, or a plant's natural defenses to being chemically dismantled. Adding to the complexity is the fact biofuel production that involves several steps—pretreatment, enzyme treatment and fermentation—is more costly than a method that combines biomass utilization and the fermentation of sugars to biofuel into a single process.
To make the conversion possible, researchers had to develop a strain of Clostridium cellulolyticum, a native cellulose-degrading microbe, that could synthesize isobutanol directly from cellulose. This proof of concept research sets the stage for studies that will likely involve genetic manipulation of other consolidated bioprocessing microorganisms.[10]
Cyanobacteria
Cyanobacteria, are a phylum of photosynthetic bacteria.[11] Cyanobacteria are suited for isobutanol biosynthesis when genetically engineered to produce isobutanol and its corresponding aldehydes.[12] Isobutanol producing species of cyanobacteria offer several advantages as biofuel synthesizers:
- Cyanobacteria grow faster than plants[13] and also absorb sunlight more efficiently than plants.[14] This means they can be replenished at a faster rate than the plant matter used for other biofuel biosynthesizers.
- Cyanobacteria can be grown on non-arable land (land not used for farming).[13] This prevents competition between food sources and fuel sources.[13]
- The supplements necessary for the growth of Cyanobacteria are CO2, H2O, and sunlight.[14] This presents two advantages:
- Because CO2 is derived from the atmosphere, Cyanobacteria do not need plant matter to synthesize isobutanol (in other organisms which synthesize isobutanol, plant matter is the source of the carbon necessary to synthetically assemble isobutanol).[14] Since plant matter is not used by this method of isobutanol production, the necessity to source plant matter from food sources and create a food-fuel price relationship is avoided.[13]
- Because CO2 is absorbed from the atmosphere by Cyanobacteria, the possibility of bioremediation (in the form of Cyanobacteria removing excess CO2 from the atmosphere) exists.[14]
The primary drawbacks of Cyanobacteria are:
- Cyanobacteria are sensitive to environmental conditions when being grown. Cyanobacteria suffer greatly from sunlight of inappropriate wavelength and intensity, CO2 of inappropriate concentration, or H2O of inappropriate salinity though a wealth of cyanobacteria are able to grow in brackish and marine waters. These factors are generally hard to control, and present a major obstacle in cyanbacterial production of isobutanol.[15]
- Cyanobacteria bioreactors require high energy to operate. Cultures require constant mixing, and the harvesting of biosynthetic products is energy intensive. This reduces the efficiency of isobutanol production via Cyanobacteria.[15]
Bacillus subtilis
Bacillus subtilis is a gram-positive rod-shaped bacteria. Bacillus subtilis offers many of the same advantages and disadvantages of E. coli, but it is less prominently used and does not produce isobutanol in quantities as large as E. coli.[7] Similar to E. coli, Bacillus subtilis is capable of producing isobutanol from lignocellulose, and is easily manipulated by common genetic techniques.[7] Elementary mode analysis has also been used to improve the isobutanol-synthesis metabolic pathway used by Bacillus subtilis, leading to higher yields of isobutanol being produced.[16]
Saccharomyces cerevisiae
Saccharomyces cerevisiae, or S. cerevisiae is a species of yeast. S. cerevisiae naturally produces isobutanol in small quantities via its valine biosynthetic pathway.[17] S. cerevisiae is an ideal candidate for isobutanol biofuel production for several reasons:
- S. cerevisiae can be grown at low pH levels, helping prevent contamination during growth in industrial bioreactors.[7]
- S. cerevisiae cannot be affected by bacteriophages because it is a eukaryote.[7]
- Extensive scientific knowledge about S. cerevisiae and its biology already exists.[7]
Overexpression of the enzymes in the valine biosynthetic pathway of S. cerevisiae has been used to improve isobutanol yields.[17][18][19] S. cerevisiae, however, has proved difficult to work with because of its inherent biology:
- As a eukaryote, S. cerevisiaeis genetically more complex than E. coli or B. subtilis, and is harder to genetically manipulate as a result.[7]
- S. cerevisiae has the natural ability to produce ethanol. This natural ability can "overpower" and consequently inhibit isobutanol production by S. cerevisiae.[7]
- S. cerevisiae cannot use five carbon sugars to produce isobutanol. The inability to use five-carbon sugars restricts S. cerevisiae from using lignocellulose, and means S. cerevisiae must use plant matter intended for human consumption to produce isobutanol. This results in an unfavorable food/fuel price relationship when isobutanol is produced by S. cerevisiae.[7]
Ralstonia eutropha
Ralstonia eutropha is a gram-negative soil bacterium of the betaproteobacteria class. Ralstonia eutropha is capable of converting electrical energy into isobutanol. This conversion is completed in several steps:
- Anodes are placed in a mixture of H2O and CO2.
- An electric current is run through the anodes, and through an electrochemical process H2O and CO2 are combined to synthesize formic acid.
- A culture of Ralstonia eutropha (composed of a strain tolerant to electricity) is kept within the H2O and CO2 mixture.
- The culture of Ralstonia eutropha then converts formic acid from the mixture into isobutanol.
- The biosynthesized isobutanol is then separated from the mixture, and can be used as a biofuel.
This method of isobutanol production offers a way to chemically store energy produced from sustainable sources.[20]
Applications
Isobutanol has a variety of technical and industrial applications:
- feedstock in the manufacture of isobutyl acetate, which is used in the production of lacquer and similar coatings, and in the food industry as a flavoring agent
- precursor of derivative esters - isobutyl esters such as diisobutyl phthalate (DIBP) are used as plasticizers in plastics, rubbers, and other dispersions
- precursor of p-xylene, a building block for plastic bottles, textiles and clothing.
- paint solvent
- varnish remover
- ink ingredient
- paint additive, to reduce viscosity, improve brush flow, and retard formation of oil residues (blush) on painted surfaces
- gasoline additive, to reduce carburetor icing
- automotive polish additive
- automotive paint cleaner additive
- chemical extractant in production of organic compounds
- mobile phase in thin layer chromatography.
- potential gasoline alternative
Second-generation biofuel
Isobutanol can be used as a biofuel substitute for gasoline in the current petroleum infrastructure. Isobutanol has not yet been put into mainstream use as a biofuel and would serve as a replacement for ethanol. Ethanol is a first-generation biofuel, and is used primarily as a gasoline additive in the petroleum infrastructure. Isobutanol is a second-generation biofuel with several qualities that resolve issues presented by ethanol.[7]
Isobutanol's properties make it an attractive biofuel:[7]
- relatively high energy density, 98% of that of gasoline.[21]
- does not readily absorb water from air, preventing the corrosion of engines and pipelines.[7]
- can be mixed at any proportion with gasoline,[22] meaning the fuel can "drop into" the existing petroleum infrastructure as a replacement fuel or major additive.[7]
- can be produced from plant matter not connected to food supplies, preventing a fuel-price/food-price relationship.[7][8][9][16]
Safety and regulation
Isobutanol is one of the least toxic of the butanols with an LD50 of 2460 mg/kg (rat, oral).
In March 2009, the Canadian government announced a ban on isobutanol use in cosmetics.[23]
See also
References
- 1 2 Isobutanol, International Chemical Safety Card 0113, Geneva: International Programme on Chemical Safety, April 2005.
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0352". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Iso-butanol". ChemicalLand21.
- ↑ "Isobutyl alcohol". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Hahn, Heinz-Dieter; Dämbkes, Georg; Rupprich, Norbert (2005), "Butanols", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a04_463.
- 1 2 3 4 Atsumi, Shota; Hanai, Taizo; Liao, James C. "Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels". Nature. 451 (7174): 86–89. doi:10.1038/nature06450.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Peralta-Yahya, Pamela P.; Zhang, Fuzhong; del Cardayre, Stephen B.; Keasling, Jay D.; Del Cardayre, Stephen B.; Keasling, Jay D. (15 August 2012). "Microbial engineering for the production of advanced biofuels". Nature. 488 (7411): 320–328. Bibcode:2012Natur.488..320P. doi:10.1038/nature11478. PMID 22895337.
- 1 2 Trinh, Cong T. (9 June 2012). "Elucidating and reprogramming Escherichia coli metabolisms for obligate anaerobic n-butanol and isobutanol production". Applied Microbiology and Biotechnology. 95 (4): 1083–1094. doi:10.1007/s00253-012-4197-7. PMID 22678028.
- 1 2 Nakashima, Nobutaka; Tamura, Tomohiro (1 July 2012). "A new carbon catabolite repression mutation of Escherichia coli, mlc∗, and its use for producing isobutanol". Journal of Bioscience and Bioengineering. 114 (1): 38–44. doi:10.1016/j.jbiosc.2012.02.029. PMID 22561880.
- ↑ Higashide, Wendy; Li, Yongchao; Yang, Yunfeng; Liao, James C. (2011-04-15). "Metabolic Engineering of Clostridium cellulolyticum for Production of Isobutanol from Cellulose". Applied and Environmental Microbiology. 77 (8): 2727–2733. doi:10.1128/AEM.02454-10. ISSN 0099-2240. PMC 3126361
 . PMID 21378054.
. PMID 21378054. - ↑ Cyanobacteria
- ↑ Atsumi, Shota; Higashide, Wendy; Liao, James C. "Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde". Nature Biotechnology. 27 (12): 1177–1180. doi:10.1038/nbt.1586.
- 1 2 3 4 Machado, Iara M.P.; Atsumi, Shota (1 November 2012). "Cyanobacterial biofuel production". Journal of Biotechnology. 162 (1): 50–56. doi:10.1016/j.jbiotec.2012.03.005. PMID 22446641.
- 1 2 3 4 Varman, A. M.; Xiao, Y.; Pakrasi, H. B.; Tang, Y. J. (26 November 2012). "Metabolic Engineering of Synechocystis sp. Strain PCC 6803 for Isobutanol Production". Applied and Environmental Microbiology. 79 (3): 908–914. doi:10.1128/AEM.02827-12. PMID 23183979.
- 1 2 Singh, Nirbhay Kumar; Dhar, Dolly Wattal (11 March 2011). "Microalgae as second generation biofuel. A review". Agronomy for Sustainable Development. 31 (4): 605–629. doi:10.1007/s13593-011-0018-0.
- 1 2 Li, Shanshan; Huang, Di; Li, Yong; Wen, Jianping; Jia, Xiaoqiang (1 January 2012). "Rational improvement of the engineered isobutanol-producing Bacillus subtilis by elementary mode analysis". Microbial Cell Factories. 11 (1): 101. doi:10.1186/1475-2859-11-101.
- 1 2 Kondo, Takashi; Tezuka, Hironori; Ishii, Jun; Matsuda, Fumio; Ogino, Chiaki; Kondo, Akihiko (1 May 2012). "Genetic engineering to enhance the Ehrlich pathway and alter carbon flux for increased isobutanol production from glucose by Saccharomyces cerevisiae". Journal of Biotechnology. 159 (1–2): 32–37. doi:10.1016/j.jbiotec.2012.01.022. PMID 22342368.
- ↑ MATSUDA, Fumio; KONDO, Takashi; IDA, Kengo; TEZUKA, Hironori; ISHII, Jun; KONDO, Akihiko (1 January 2012). "Construction of an Artificial Pathway for Isobutanol Biosynthesis in the Cytosol of Saccharomyces cerevisiae". Bioscience, Biotechnology, and Biochemistry. 76 (11): 2139–2141. doi:10.1271/bbb.120420.
- ↑ Lee, Won-Heong; Seo, Seung-Oh; Bae, Yi-Hyun; Nan, Hong; Jin, Yong-Su; Seo, Jin-Ho (28 April 2012). "Isobutanol production in engineered Saccharomyces cerevisiae by overexpression of 2-ketoisovalerate decarboxylase and valine biosynthetic enzymes". Bioprocess and Biosystems Engineering. 35 (9): 1467–1475. doi:10.1007/s00449-012-0736-y. PMID 22543927.
- ↑ Li, H.; Opgenorth, P. H.; Wernick, D. G.; Rogers, S.; Wu, T.-Y.; Higashide, W.; Malati, P.; Huo, Y.-X.; Cho, K. M.; Liao, J. C. (29 March 2012). "Integrated Electromicrobial Conversion of CO2 to Higher Alcohols". Science. 335 (6076): 1596–1596. Bibcode:2012Sci...335.1596L. doi:10.1126/science.1217643.
- ↑ Lu, Jingnan; Brigham, Christopher J.; Gai, Claudia S.; Sinskey, Anthony J. (4 August 2012). "Studies on the production of branched-chain alcohols in engineered Ralstonia eutropha". Applied Microbiology and Biotechnology. 96 (1): 283–297. doi:10.1007/s00253-012-4320-9. PMID 22864971.
- ↑ Ting, Cindy Ng Wei; Wu, Jinchuan; Takahashi, Katsuyuki; Endo, Ayako; Zhao, Hua (8 September 2012). "Screened Butanol-Tolerant Enterococcus faecium Capable of Butanol Production". Applied Biochemistry and Biotechnology. 168 (6): 1672–1680. doi:10.1007/s12010-012-9888-0. PMID 22961352.
- ↑ "Cosmetic Chemicals Banned in Canada", Chem. Eng. News, 87 (11): 38, 2009-03-16.
External links
| Wikimedia Commons has media related to Isobutanol. |
- International Chemical Safety Card 0113
- "NIOSH Pocket Guide to Chemical Hazards #0352". National Institute for Occupational Safety and Health (NIOSH).
- IPCS Environmental Health Criteria 65: Butanols: four isomers
- IPCS Health and Safety Guide 9: Isobutanol
