Cinolazepam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Gerodorm |
| AHFS/Drugs.com | cinolazepam |
| Routes of administration | Oral |
| ATC code | N05CD13 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90–100% |
| Metabolism | Hepatic |
| Biological half-life | 9 hours[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
75696-02-5 |
| PubChem (CID) | 3033621 |
| DrugBank |
DB01594 |
| ChemSpider |
2298251 |
| UNII |
68P0556B0U |
| KEGG |
D07328 |
| ChEBI |
CHEBI:59514 |
| ChEMBL |
CHEMBL2104926 |
| Chemical and physical data | |
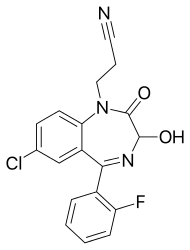
| Formula | C18H13ClFN3O2 |
| Molar mass | 357.8 |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| | |
Cinolazepam[2] (marketed under the brand name Gerodorm)[3] is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Due to its strong sedative properties, it is primarily used as an hypnotic.
Cinolazepam is not approved for sale in the United States or Canada.
See also
References
- ↑ Saletu, B.; G. Kindshofer; P. Anderer; J. Grunberger (1987). "Short-term sleep laboratory studies with cinolazepam in situational insomnia induced by traffic noise.". International Journal of Clinical Pharmacology Research. 7 (5): 407–18. PMID 2889679.
- ↑ US Patent 4388313 Novel 3-hydroxy-1,4-benzodiazepine-2-ones and process for the preparation thereof
- ↑ Lannacher Romania (1999). "Gerodorm". Produse Gerot inregistrate in Romania (in Romanian). Archived from the original on 11 October 2006. Retrieved 17 August 2006.
External links
This article is issued from Wikipedia - version of the 11/25/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.