Pazinaclone
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
103255-66-9 |
| PubChem (CID) | 59743 |
| ChemSpider |
53893 |
| UNII |
MHK03047IJ |
| KEGG |
D05378 |
| ChEMBL |
CHEMBL2107504 |
| Chemical and physical data | |
| Formula | C25H23ClN4O4 |
| Molar mass | 478.928 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Pazinaclone (DN-2327) is a sedative and anxiolytic drug in the cyclopyrrolone family of drugs. Some other cyclopyrrolone drugs include zopiclone and eszopiclone.
Pazinaclone has a very similar pharmacological profile to the benzodiazepines including sedative and anxiolytic properties, but with less amnestic effects,[1] and at low doses it is a relatively selective anxiolytic, with sedative effects only appearing at higher doses.[2]
Pazinaclone produces its sedative and anxiolytic effects by acting as a partial agonist at GABAA benzodiazepine receptors, although pazinaclone is more subtype-selective than most benzodiazepines.[3]
Synthesis
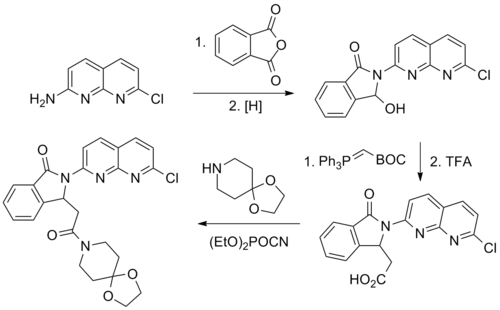
Reaction of 2-amino-7-chloro-1,8-naphthyridine with phthalic anhydride leads to the corresponding phthalimide. Selective reduction of one of the imide carbonyl groups in essence converts that to an aldehyde. Condensation with tert-butyl(triphenylphosphoranylidene)acetate gives the Wittig product.
The carboxylic acid is then treated with diethyl cyanophosphonate to convert that to an activated acid cyanide; reaction with 1,4-dioxa-8-azaspiro[4.5]decane results in formation of the corresponding amide, pazinaclone.
See also
References
- ↑ Wada T, Fukuda N. Effect of a new anxiolytic, DN-2327, on learning and memory in rats. Pharmacology, Biochemistry and Behavior. 1992 Mar;41(3):573-9.
- ↑ Suzuki M, Uchiumi M, Murasaki M. A comparative study of the psychological effects of DN-2327, a partial benzodiazepine agonist, and alprazolam. Psychopharmacology (Berlin). 1995 Oct;121(4):442-50.
- ↑ Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opinion on Investigational Drugs. 2005 May;14(5):601-18.