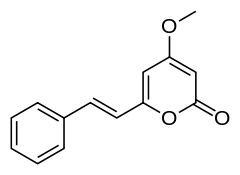
Desmethoxyyangonin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-methoxy-6-[(E)-2-phenylethenyl]-2H-pyran-2-one | |
| Other names
5,6-Dehydrokawain 4-methoxy-6-[(E)-2-phenylvinyl]-2-pyranone | |
| Identifiers | |
| 15345-89-8 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL254218 |
| ChemSpider | 4438012 |
| PubChem | 5273621 |
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.25 g·mol−1 |
| Appearance | white to faint yellow powder |
| Density | 1.18 g/mL |
| Melting point | 148 °C (298 °F; 421 K) |
| Boiling point | 440 °C (824 °F; 713 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Desmethoxyyangonin or 5,6-dehydrokawain is one of the six major kavalactones found in the Piper methysticum (kava) plant.
Pharmacology
Desmethoxyyangonin is a reversible inhibitor of monoamine oxidase B (MAO-B).[1] Kava is able to increase dopamine levels in the nucleus accumbens[2] and desmethoxyyangonin likely contributes to this effect. This, along with the potential increases of serotonin and other catecholamine concentrations, may be responsible for the purported attention-promoting effects of kava.
Desmethoxyyangonin has marked activity on the induction of CYP3A23.[3]
See also
References
- ↑ Uebelhack, R; Franke L; Schewe HL (September 1998). "Inhibition of platelet MAO-B by kava pyrone-enriched extract from Piper methysticum Forster (kava-kava)". Pharmacopsychiatry. 31 (5): 187–192. doi:10.1055/s-2007-979325. PMID 9832350.
- ↑ Baum, SS; Hill R; Rommelspacher H (October 1998). "Effect of kava extract and individual kavapyrones on neurotransmitter levels in the nucleus accumbens of rats". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 22 (7): 1105–1120. doi:10.1016/S0278-5846(98)00062-1. PMID 9829291.
- ↑ Ma, Yuzhong; Karuna Sachdeva; Jirong Liu1; Michael Ford; Dongfang Yang; Ikhlas Khan; Clinton Chichester; Bingfang Yan (November 2004). "Desmethoxyyangonin and dihydromethysticin are two major pharmacological kavalactones with marked activity on the induction of CYP3A23". Drug Metabolism and Disposition. 32 (11): 1317–1324. doi:10.1124/dmd.104.000786. PMID 15282211.
This article is issued from Wikipedia - version of the 2/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.