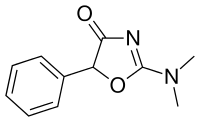
Thozalinone
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | Tozalinone, Thozalinon |
| CAS Number | 655-05-0 |
| PubChem (CID) | 12602 |
| ChemSpider |
12082 |
| UNII |
68X5932947 |
| KEGG |
D06115 |
| Chemical and physical data | |
| Formula | C11H12N2O2 |
| Molar mass | 204.225 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Thozalinone (USAN) (brand name Stimsen; former developmental code name CL-39808) is a psychostimulant that has been used as an antidepressant in Europe.[1][2][3][4][5] It has also been trialed as an anorectic.[6] Thozalinone is described as a "dopaminergic stimulant",[7] and likely acts via inducing the release of dopamine and norepinephrine similarly to its analogues pemoline and aminorex.[2][8][9]
See also
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 435–. ISBN 978-1-4757-2085-3.
- 1 2 Greenblatt EN, Osterberg AC (July 1965). "Some pharmacologic properties of thozalinone, a new excitant". Toxicology and Applied Pharmacology. 7 (4): 566–78. doi:10.1016/0041-008X(65)90042-6. PMID 4378772.
- ↑ Dictionary of organic compounds. London: Chapman & Hall. 1996. ISBN 0-412-54090-8.
- ↑ Merck index on CD-ROM: Windows. London: Chapman & Hall EPD. 1998. ISBN 0-412-82910-X.
- ↑ Gallant DM, Bishop MP, Scrignar CB, Hornsby L, Moore B, Inturrisi BB (December 1966). "A double-blind study of thozalinone (C1 39,808) in depressed outpatients". Current Therapeutic Research, Clinical and Experimental. 8 (12): 621–2. PMID 4962734.
- ↑ Leite AC, Liepen LL, Costa VP (September 1971). "[Clinical trial of Stimsem Thozalinone in the treatment of obese patients]". Revista Brasileira De Medicina (in Portuguese). 28 (9): 475–8. PMID 5139648.
- ↑ Yen-koo, H. C.; Balazs, T. (2015). "Detection of Dopaminergic Supersensitivity Induced by Neuroleptic Drugs in Mice". Drug and Chemical Toxicology. 3 (2): 237–247. doi:10.3109/01480548009108286. ISSN 0148-0545.
- ↑ Yen-Koo HC, Balazs T (1980). "Detection of dopaminergic supersensitivity induced by neuroleptic drugs in mice". Drug and Chemical Toxicology. 3 (2): 237–47. doi:10.3109/01480548009108286. PMID 6112126.
- ↑ Yen-Koo HC, Davis DA, Balazs T (1985). "Inhibition of dopaminergic agonist-induced gnawing behavior by neuroleptic drugs in mice". Drug and Chemical Toxicology. 8 (6): 495–502. doi:10.3109/01480548509041072. PMID 2868876.
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| Central |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral | |||||||||||
| |||||||||||
| α1 |
| ||||||
|---|---|---|---|---|---|---|---|
| α2 |
| ||||||
| β |
| ||||||
| |||||||
| D1-like |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the 9/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.