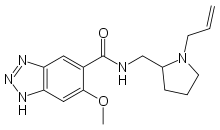
Alizapride
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, IM, IV |
| ATC code | A03FA05 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 3 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
59338-93-1 |
| PubChem (CID) | 43008 |
| DrugBank |
DB01425 |
| ChemSpider |
39202 |
| UNII |
P55703ZRZY |
| KEGG |
D07102 |
| ChEMBL |
CHEMBL290194 |
| Chemical and physical data | |
| Formula | C16H21N5O2 |
| Molar mass | 315.37 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Alizapride (Litican, Plitican, Superan, Vergentan) is a dopamine antagonist with prokinetic and antiemetic effects used in the treatment of nausea and vomiting, including postoperative nausea and vomiting. It is structurally related to metoclopramide and other benzamides.[1]
References
- ↑ Bleiberg H, Gerard B, Dalesio O, Crespeigne N, Rozencweig M (1988). "Activity of a new antiemetic agent: alizapride. A randomized double-blind crossover controlled trial". Cancer Chemother Pharmacol. 22 (4): 316–20. doi:10.1007/bf00254238. PMID 3048762.
This article is issued from Wikipedia - version of the 6/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.