Guar gum
 | |
| Identifiers | |
|---|---|
| 9000-30-0 | |
| ECHA InfoCard | 100.029.567 |
| E number | E412 (thickeners, ...) |
| Properties | |
| Density | 0.8-1.0 g/mL at 25 °C |
| Acidity (pKa) | 5-7 |
| Pharmacology | |
| A10BX01 (WHO) | |
| Hazards | |
| Safety data sheet | MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Guar gum, also called guaran, is a galactomannan. It is primarily the ground endosperm of guar beans. The guar seeds are dehusked, milled and screened to obtain the guar gum.[1] It is typically produced as a free-flowing, off-white powder.
Production and trade
The guar bean is principally grown in India, Pakistan, U.S., Australia and Africa. India produces 2.5 - 3.5 million tons of guar annually, making it the largest producer with about 80% of world production, while Pakistan produced 250,000 tons of guar in 2013.
In India, Rajasthan, Gujarat and Haryana are the main producing regions, and Jodhpur, Sri Ganganagar and Hanumangarh in Rajasthan are the major Guar trading markets. The best quality guar beans are sometimes known as "Fast-Hydrating Guar".
The United States has produced 4,600 to 14,000 tonnes of guar over the last 5 years.[2] As many as 50,000 acres of guar have been grown in West Texas over the last 50 years.
The world production for guar gum and its derivatives is about 1.0 Million tonnes. Industrial guar gum accounts for about 70% of the total demand. Mainly it is used as a proppant transport / proppant suspending agent in Hydraulic Fracturing Process. In 2012 guar prices increased by 900-1000%. At its peak it reached to $28000 per ton. however later stabilised to $8000 per ton. The main reason for this large scale price rise was the inventory build up by companies like Halliburton and Schlumberger, Baker Hughes, Calfrac Well Services, amidst the fear of shortage of guar gum for drilling due to ongoing drought in Rajasthan. 2013 was a strong year for guar sowing and production in India. The total sowing area rose by 21 percent in 2013 to reach 10.6 million acres. Rajasthan, Haryana, and Gujarat – the three key guar-producing states – exceeded the sowing area target set by their respective agriculture departments. Non-traditional guar cultivators in other Indian states also showed keen interest in the crop in 2013.[3][4] Calgary-based Trican Well Services Ltd. touts its trademarked guar substitutes TriFrac-C and Novum. Baker Hughes trademarked something called “AquaPerm,” while Halliburton rolled out “PermStim” . By 2013, Schlumberger was advertising its trademarked guar-substitute, “HiWay.” Most of these laboratory substitutes use biodegradable polymers.
But according to market trends analyst Thomasnet.com (May 9, 2013), “…there isn’t anything currently available with the reliability and quantities of guar gum.”
The American Petroleum Institute’s July 2014 report, Hydraulic Fracturing: Unlocking America’s Natural Gas Resources, uses images of a tube of lipstick and an ice cream bar (which both contain guar gum) as examples of the nonthreatening ingredients in fracking fluids. One of India’s biggest guar exporters, Vikas WSP, gave away 3,000 tons of guar seeds to encourage farmers to switch away from cotton and other crops to cultivation of guar bushes.
Properties
Chemical composition
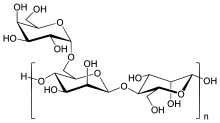
Chemically, guar gum is a polysaccharide composed of the sugars galactose and mannose. The backbone is a linear chain of β 1,4-linked mannose residues to which galactose residues are 1,6-linked at every second mannose, forming short side-branches.
Solubility and viscosity
Guar gum is more soluble than locust bean gum, as it has more galactose branch points. Unlike locust bean gum, it is not self-gelling.[5] However, either borax or calcium can cross-link guar gum, causing it to gel. In water, it is nonionic and hydrocolloidal. It is not affected by ionic strength or pH, but will degrade at extreme pH and temperature (e.g. pH 3 at 50 °C).[5] It remains stable in solution over pH range 5-7. Strong acids cause hydrolysis and loss of viscosity, and alkalies in strong concentration also tend to reduce viscosity. It is insoluble in most hydrocarbon solvents. The viscosity attained is dependent on time, temperature, concentration, pH, rate of agitation and practical size of the powdered gum used. The lower the temperature lower the rate at which viscosity increases and the lower the final viscosity.
Above 80° the final viscosity is slightly reduced. The finer guar powders swells more rapidly than coarse powdered gum.
Guar gum shows a clear low shear plateau on the flow curve and is strongly shear thinning. The rheology of guar gum is typical for a random coil polymer. It does not show the very high low shear plateau viscosities seen with more rigid polymer chains such as xanthan gum. It is very thixotropic above 1% concentration, but below 0.3%, the thixotropy is slight. Guar gum shows viscosity synergy with xanthan gum. Guar gum and micellar casein mixtures can be slightly thixotropic if a biphase system forms.[5][6]
Thickening
Guar gum is economical because it has almost eight times the water-thickening potency of cornstarch - only a very small quantity is needed for producing sufficient viscosity. Thus, it can be used in various multiphase formulations: as an emulsifier because it helps to prevent oil droplets from coalescing, and/or as a stabilizer because it helps to prevent solid particles from settling. Guar gum is a viscosifier with very favorable rheological properties. It has a really useful ability to form breakable gels when cross-linked with boron. This makes it extremely valuable for hydraulic fracturing.
Fracking entails the pumping of sand-laden fluids into an oil or natural gas reservoir at a high pressure and flow rate. This cracks the reservoir rock and then propels the cracks open. Water alone is too thin to be effective at carrying proppant sand, so guar gum is one of the ingredients added to thicken the slurry mixture and improve its ability to carry proppant. There are several properties which are important 1. Thixotropic: the fluid should be thixotropic, meaning it should gel within few hours. 2. Gelling and degelling: The desired viscosity changes over the course of a few hours. When the fracking slurry is mixed, it needs to be thin enough to make it easier to pump. Then as it flows down the pipe, the fluid needs to gel up to support the proppant and flush it deep into the fractures. After that process, the gel has to break down so we can flow back and recover the fracking fluid, but leave the proppant behind. This requires a chemical process which produces then breaks the gel cross-linking at a predictable rate.
guar+boron+proprietary chemicals can accomplish both of these goals at once.
Ice crystal growth
Guar gum retards ice crystal growth nonspecifically by slowing mass transfer across the solid/liquid interface. It shows good stability during freeze-thaw cycles. It is therefore used in gluten free ice cream. Guar gum has synergistic effects with locust bean gum and sodium alginate. May be synergistic with xanthan: together with xanthan gum, it produces a thicker product (0.5% guar gum / 0.35% xanthan gum), which is used in applications such as soups, which do not require clear results.
Guar gum is a hydrocolloid, which is particularly useful for making thick pastes without forming a gel, and for keeping water bound in a sauce or emulsion. Guar gum can be used for thickening cold and hot liquids, to make hot gels, light foams and as an emulsion stabilizer. Guar gum can be used for cottage cheeses, curds, yogurt, sauces, soups and frozen desserts. Guar gum is also a good source of fiber with 80% soluble dietary fiber on a dry weight basis.[5]
Grading
Guar gum is analysed for
| Test | Test Method | Test | Test method |
|---|---|---|---|
| Colour | TP/09 | Acid-insoluble residue | TP/115 |
| Viscosity | TP/10/04 | Fat content | TP/18 |
| Granulation (mesh) | TP/21 | Ash content | TP/12 |
| Moisture, pH | TP/1 and TP/29 | Gum content | TP/03 |
| Protein | TP/05 | Heavy metals | TP/13 |
| Insolubles Ash | TP/11 | Filterability | TP/20A |
Guar gum powder standards are:
- HS-Code- 130 232 30
- CAS No.- 9000-30-0
- EEC No.- E 412
- BT No.- 1302 3290
- EINECS No. - 232-536-8
- Imco Code- Harmless
Manufacturing process
Depending upon the requirement of end product, various processing techniques are used. The commercial production of guar gum normally uses roasting, differential attrition, sieving, and polishing.
Food-grade guar gum is manufactured in stages. Guar split selection is important in this process. The split is screened to clean it and then soaked to prehydrate it in a double-cone mixer. The prehydrating stage is very important because it determines the rate of hydration of the final product.
The soaked splits, which have reasonably high moisture content, are passed through a flaker. The flaked guar split is ground and then dried. The powder is screened through rotary screens to deliver the required particle size. Oversize particles are either recycled to main ultra fine or reground in a separate regrind plant, according to the viscosity requirement.
This stage helps to reduce the load at the grinder. The soaked splits are difficult to grind. Direct grinding of those generates more heat in the grinder, which is not desired in the process, as it reduces the hydration of the product. Through the heating, grinding, and polishing process, the husk is separated from the endosperm halves and the refined guar split is obtained. Through the further grinding process, the refined guar split is then treated and converted into powder.
The split manufacturing process yields husk and germ called “guar meal”, widely sold in the international market as cattle feed. It is high in protein and contains oil and albuminoids, about 50% in germ and about 25% in husks. The quality of the food-grade guar gum powder is defined from its particle size, rate of hydration, and microbial content.
Manufacturers define different grades and qualities of guar gum by the particle size, the viscosity generated with a given concentration, and the rate at which that viscosity develops. Coarse-mesh guar gums will typically, but not always, develop viscosity more slowly. They may achieve a reasonably high viscosity, but will take longer to achieve. On the other hand, they will disperse better than fine-mesh, all conditions being equal. A finer mesh, such as a 200 mesh, requires more effort to dissolve.[7]
Modified forms of guar gum are available commercially, including enzyme-modified, cationic and hydropropyl guar.[8]
Industrial applications
- Textile industry – sizing, finishing and printing
- Paper industry – improved sheet formation, folding and denser surface for printing
- Explosives industry – as waterproofing agent mixed with ammonium nitrate, nitroglycerin, etc.
- Pharmaceutical industry – as binder or as disintegrator in tablets; main ingredient in some bulk-forming laxatives
- Cosmetics and toiletries industries – thickener in toothpastes, conditioner in shampoos (usually in a chemically modified version)
- Hydraulic fracturing Shale oil and gas extraction industries consumes about 90% of guar gum produced from India and Pakistan.[9]
Fracturing fluids normally consist of many additives that serve two main purposes, firstly to enhance fracture creation and proppant carrying capability and secondly to minimize formation damage. Viscosifiers, such as polymers and crosslinking agents, temperature stabilizers, pH control agents, and fluid loss control materials are among the additives that assist fracture creation. Formation damage is minimized by incorporating breakers, biocides, and surfactants. More appropriate gelling agents are linear polysaccharides, such as guar gum, cellulose, and their derivatives.
Guar Gum and Guar Derivatives in Fracturing
Guar gums are preferred as thickeners for Enhanced Oil Recovery (EOR), guar gum and its derivatives account for most of the gelled fracturing fluids. Guar is more water-soluble than other gums, and it is also a better emulsifier, because it has more galactose branch points. Guar gum shows high low-shear viscosity, but it is strongly shear-thinning. Being non-ionic, it is not affected by ionic strength or pH but will degrade at low pH at moderate temperature (pH 3 at 50 °C). Guar's derivatives demonstrate stability in high temperature and pH environments. Guar use allows for achieving exceptionally high viscosities, which improves the ability of the fracturing liquid to transport proppant. Guar hydrates fairly rapidly in cold water to give highly viscous pseudoplastic solutions of, generally, greater low-shear viscosity than other hydrocolloids. The colloidal solids present in guar make fluids more efficient by creating less filter cake. Proppant pack conductivity is maintained by utilizing a fluid that has excellent fluid loss control, such as the colloidal solids present in guar gum.
Guar has up to eight times the thickening power of starch. Derivatization of guar gum leads to subtle changes in properties, such as, decreased hydrogen bonding, increased solubility in water-alcohol mixture, and improved electrolyte compatibility. These changes in properties result in increased use in different fields, like textile printing, explosives, and oil-water fracturing applications.
Crosslinking Guar
Guar molecules have a tendency to aggregate during the hydraulic fracturing process, mainly due to intermolecular hydrogen bonding. These aggregates are detrimental to oil recovery because they clog the fractures, restricting the flow of oil. Cross-linking guar polymer chains prevents aggregation by forming metal – hydroxyl complexes. The first crosslinked guar gels were developed in the late ‘60’s. Several metal additives have been used for crosslinking, among them are chromium, aluminum, antimony, zirconium, and the more commonly used, boron. Boron, in the form of B(OH)4, reacts with the hydroxyl groups on the polymer in a two step process to link two polymer strands together to form bis-diol complexes .
1:1 1,2 diol complex and a 1:1 1,3 diol complex, place the negatively charged borate ion onto the polymer chain as a pendant group. Boric acid itself does not apparently complex to the polymer so that all bound boron is negatively charged. The primary form of crosslinking may be due to ionic association between the anionic borate complex and adsorbed cations on the second polymer chain . The development of cross-linked gels was a major advance in fracturing fluid technology. Viscosity is enhanced by tying together the low molecular weight strands, effectively yielding higher molecular weight strands and a rigid structure. Cross-linking agents are added to linear polysaccharide slurries to provide higher proppant transport performance, relative to linear gels .
Lower concentrations of guar gelling agents are needed when linear guar chains are cross-linked. It has been determined that reduced guar concentrations provide better and more complete breaks in a fracture. The breakdown of cross-linked guar gel after the fracturing process restores formation permeability and allows increased production flow of petroleum products .
- Mining
- Hydroseeding – formation of seed-bearing "guar tack"[10]
- Medical institutions, especially nursing homes - used to thicken liquids and foods for patients with dysphagia
- Fire retardant industry - as a thickener in Phos-Chek
- Nanoparticles industry - to produce silver or gold nanoparticles, or develop innovative medicine delivery mechanisms for drugs in pharmaceutical industry.
Food applications
The largest market for guar gum is in the food industry. In the US, differing percentages are set for its allowable concentration in various food applications.[11][12] In Europe, guar gum has EU food additive code E412. Xanthan gum and guar gum are the most frequently used gums in gluten-free recipes and gluten-free products.
Applications include:
- In baked goods, it increases dough yield, gives greater resiliency, and improves texture and shelf life; in pastry fillings, it prevents "weeping" (syneresis) of the water in the filling, keeping the pastry crust crisp. It is primarily used in hypoallergenic recipes that use different types of whole-grain flours. Because the consistency of these flours allows the escape of gas released by leavening, guar gum is needed to improve the thickness of these flours, allowing them to rise as a normal flour would.[13]
- In dairy products, it thickens milk, yogurt, kefir, and liquid cheese products, and helps maintain homogeneity and texture of ice creams and sherbets. It is used for similar purposes in plant milks.
- For meat, it functions as a binder.
- In condiments, it improves the stability and appearance of salad dressings, barbecue sauces, relishes, ketchups and others.
- In canned soup, it is used as a thickener and stabilizer.
- It is also used in dry soups, instant oatmeal, sweet desserts, canned fish in sauce, frozen food items, and animal feed.
- The FDA has banned guar gum as a weight loss pill due to reports of the substance swelling and obstructing the intestines and esophagus.[14]
Nutritional and medicinal effects
Guar gum, as a water-soluble fiber, acts as a bulk-forming laxative, so is claimed to be effective in promoting regular bowel movements and relieving constipation and chronic related functional bowel ailments, such as diverticulosis, Crohn's disease, colitis and irritable bowel syndrome.
Several studies have found significant decreases in human serum cholesterol levels following guar gum ingestion. These decreases are thought to be a function of its high soluble fiber content.
Guar gum has been considered of interest in regard to both weight loss and diabetic diets. It is a thermogenic substance.[15] Moreover, its low digestibility lends its use in recipes as a filler, which can help to provide satiety, or slow the digestion of a meal, thus lowering the glycemic index of that meal. In the late 1980s, guar gum was used and heavily promoted in several weight-loss products. The US Food and Drug Administration eventually recalled these due to reports of esophageal blockage from insufficient fluid intake, after one brand alone caused at least 10 users to be hospitalized, and a death.[16] For this reason, guar gum is no longer approved for use in over-the-counter weight loss aids in the United States. Moreover, a meta-analysis combining the results of 11 randomized, controlled trials found guar gum supplements were not effective in reducing body weight.[17]
Guar gum, though, is also capable of reducing the absorbability of dietary minerals (other than calcium), when foods or nutritional supplements containing them are consumed concomitantly with it, but this is less of a concern with guar gum than with various insoluble dietary fibers.
Some studies have found guar gum to improve dietary glucose tolerance.[18] Research has revealed the water-soluble fiber in it may help people with diabetes by slowing the absorption of sugars by the small intestine. Although the rate of absorption is reduced, the amount of sugar absorbed is the same overall. This may help diabetic patients by moderating glucose "spikes".
Guar-based compounds, such as hydroxypropyl guar, have been in artificial tears to treat dry eye.[19]
Allergies
Some studies have found an allergic sensitivity to guar gum developed in a few individuals working in an industrial environment where airborne concentrations of the substance were present. In those affected by the inhalation of the airborne particles, common adverse reactions were occupational rhinitis and asthma.[20]
Soy protein occurs as an impurity in manufactured guar gum, and can make up as much as 10%. The guar gum can therefore adversely affect those with sensitivity to soy.[21]
A few individuals are allergic to guar gum and experience reactions such as flushing, itchiness and diarrhea.[22] Consumption for allergic individuals is also known to cause small sores or lesions.[23]
Dioxin contamination
In July 2007, the European Commission issued a health warning to its member states after high levels of dioxins were detected in a food additive - guar gum - used as thickener in small quantities in meat, dairy, dessert or delicatessen products. The source was traced to guar gum from India that was contaminated with pentachlorophenol, a pesticide no longer in use.[24] PCP contains dioxins as contamination. Dioxins damage the human immune system.[25]
Further reading
- Web page ( Provides latest update & market research on guar gum free of cost ) www.guargumcultivation.com
References
- ↑ "foa.org" (PDF). Retrieved 2011-04-18.
- ↑ Guar in West Texas http://lubbock.tamu.edu/files/2013/06/Guar-Production-Industry-Texas-May2013-Trostle.pdf
- ↑ "2014 Guar Gum Report: India".
- ↑ https://web.archive.org/web/20140408213653/http://www.guarindian.com/. Archived from the original on April 8, 2014. Retrieved March 4, 2014. Missing or empty
|title=(help) - 1 2 3 4 Martin Chaplin "Water Structure and Behavior: Guar Gum". April 2012. London South Bank University
- ↑ Lynn A. Kuntz. "Special Effects With Gums". December 1999. Food Product Design
- ↑ "Weight loss could be helped by Tummy Tuck". Retrieved 2015-09-14.
- ↑ Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, page 4770
- ↑ Ram Narayan (August 8, 2012). "From Food to Fracking: Guar Gum and International Regulation". RegBlog. University of Pennsylvania Law School. Retrieved 15 August 2012.
- ↑ "Product description: Guar Tack. S&S Seeds Inc. 2006". Ssseeds.com. Retrieved 2011-04-18.
- ↑ fda.gov- Food additive list
- ↑ Maximum Usage Levels Permitted- Guar gum
- ↑ Source: NOW Foods. Guar Gum Nutrition Label. Bloomingdale, IL: n.p., n.d.
- ↑ Lewis, JH (1992). "Esophageal and small bowel obstruction from guar gum-containing "diet pills": analysis of 26 cases reported to the Food and Drug Administration". Am. J. Gastroenterol. 87: 1424–8. PMID 1329494.
- ↑ JC Brown & G Livesey. "Energy balance and expenditure while consuming guar gum at various fat intakes and ambient temperatures". Am J Clin Nutr. 1994. 60(6):956-64 (ISSN 0002-9165)
- ↑ "Supplements: Making Sure Hype Doesnþt Overwhelm Science (original date unknown)". fda.gov. Archived from the original on 5 May 1997.
- ↑ Pittler, MH (2001). "Ernst E. Guar gum for body weight reduction: meta-analysis of randomized trials". Am J Med. 110 (9): 724–730. doi:10.1016/s0002-9343(01)00702-1.
- ↑ Daumerie C and Henquin JC (1982). "Acute effects of guar gum on glucose tolerance and intestinal absorption of nutrients in rats.". nih.gov. 8: 1–5. PMID 6284563.
- ↑ Pucker AD, Ng SM, Nichols JJ (2016). "Over the counter (OTC) artificial tear drops for dry eye syndrome". Cochrane Database Syst Rev. 2: CD009729. doi:10.1002/14651858.CD009729.pub2. PMID 26905373.
- ↑ "AllergyNet - Allergy Advisor Find". Allallergy.net. Retrieved 2013-02-19.
- ↑ "Guar Gum And Soy Allergy". Livestrong.Com. Retrieved 2013-02-19.
- ↑ "The Truth about Guar Gum". www.livescience.com. Retrieved 2012-07-27.
- ↑ "Can you be allergic to Gum?". www.livestrong.com. Retrieved 2013-08-16.
- ↑ "Commission Regulation (EU) No 258/2010". 2010-03-25. Retrieved 2012-07-14.
- ↑ "Dioxins and their effects on human health". 2010-05-01. Retrieved 2012-02-08.