Bemegride
 | |
| Clinical data | |
|---|---|
| Trade names | Mikedimide (Panray), Eukraton (Nordmark), Malysol (Arco, Switzerland), Megimide (Nicholas) |
| AHFS/Drugs.com | International Drug Names |
| ATC code | R07AB05 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms |
Methetharimide β,β-methylethylglutarimide |
| CAS Number |
64-65-3 |
| PubChem (CID) | 2310 |
| ChemSpider |
2220 |
| UNII |
57DQA39DO2 |
| KEGG |
D01957 |
| ChEMBL |
CHEMBL1214192 |
| Chemical and physical data | |
| Formula | C8H13NO2 |
| Molar mass | 155.194 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 127 °C (261 °F) |
| |
| |
| (verify) | |
Bemegride (also marketed as Megimide) is a central nervous system stimulant and antidote for barbiturate poisoning[1] as its chemoreceptor agonism increases mean tidal volume, thereby increasing respiration and the concentration of O2 in blood although it may be theoretically used as a supportive measure in treating any depressant overdose. The drug was first synthesized in 1911.[2]
As with other chemoreceptor agonists, it is a potent emetic at doses above those normally used in management of barbiturate overdose although emesis and aspiration are a concern during treatment.
John Bodkin Adams case
Bemegride is notable in legal history as the drug suspected serial killer Dr John Bodkin Adams failed to prescribe correctly to his patient Gertrude Hullett. Hullett took an overdose of barbiturates on 19 July 1956 but Adams only gave her a single 10cc dose of bemegride three days later on the 22nd, despite having acquired 100cc for her treatment. Hullett died the next day on 23 July 1956. Adams was charged but never tried for her murder.[3]
Animal use
Bemegride is also used to induce convulsions in experimental animals.[4]
Synthesis
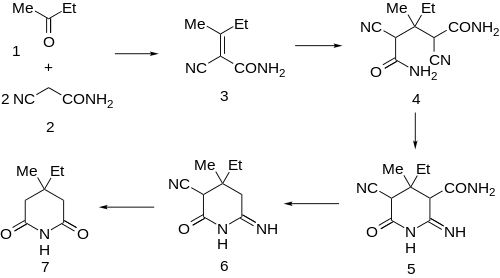
The original synthesis involves first the condensation of methylethylketone with two equivalents of cyanoacetamide. The product can be rationalized by assuming first aldol condensation of ketone and active methylene compound followed by dehydration to give 3. Conjugate addition of a second molecule of cyanoacetamide would afford 4. Addition of one of the amide amines to the nitrile would then afford the iminonitrile 5. The observed product 6 can be rationalized by assuming loss of the carboxamide under strongly basic conditions. Decarboxylative hydrolysis of 6 then leads to bemigride 7.
References
- ↑ Hofmeister, Alfred (2000). "Analeptics". Ullmann's Encyclopedia of Industrial Chemistry: 1–2. doi:10.1002/14356007.a02_267.
- 1 2 Thole, Ferdinand Bernard; Thorpe, Jocelyn Field (1911). "LIII.—The formation and reactions of iminocompounds. Part XV. The production of imino-derivatives of piperidine leading to the formation of the ββ-disubstituted glutaric acids". Journal of the Chemical Society, Transactions. 99: 422–448. doi:10.1039/CT9119900422.
- ↑ Cullen, Pamela V., A Stranger in Blood: The Case Files on Dr John Bodkin Adams, London, Elliott & Thompson, 2006, ISBN 1-904027-19-9
- ↑ Definition: bemegride from Online Medical Dictionary