5F-AB-FUPPYCA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | None |
| Chemical and physical data | |
| Formula | C20H26F2N4O2 |
| Molar mass | 392.45 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
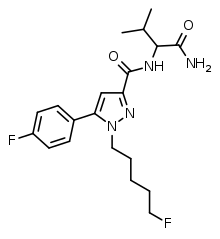
5F-AB-FUPPYCA (also known as AZ-037) is a pyrazole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug.[1][2] It was first detected by the EMCDDA as part of a seizure of 540 g white powder in France in February 2015.[3] The name AZ-037 is also used as a synonym for its structural isomer 5-fluoro-3,5-AB-PFUPPYCA on the Cayman Chemical website.[4] Thus AZ-037 is being used as a synonym for two different compounds.
See also
- 5F-3,5-AB-PFUPPYCA
- AB-CHFUPYCA
- AB-FUBINACA
- ADB-FUBINACA
- AMB-FUBINACA
- APP-FUBINACA
- FDU-NNE1
- FUB-144
- FUB-APINACA
- FUB-JWH-018
- FUB-NNE1
- FDU-PB-22
- FUB-PB-22
- MDMB-FUBICA
- MDMB-FUBINACA
References
- ↑ Girreser, Ulrich; Rösner, Peter; Vasilev, Andrej (July 2016). "Structure elucidation of the designer drug N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-3-(4-fluorophenyl)-pyrazole-5-carboxamide and the relevance of predicted 13C NMR shifts – a case study". Drug Testing and Analysis. 8 (7): 668–675. doi:10.1002/dta.1820. ISSN 1942-7611. PMID 26012418.
- ↑ Banister, Samuel D.; Longworth, Mitchell; Kevin, Richard; Sachdev, Shivani; Santiago, Marina; Stuart, Jordyn; Mack, James B. C.; Glass, Michelle; McGregor, Iain S.; Connor, Mark; Kassiou, Michael (27 July 2016). "Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues". ACS Chemical Neuroscience. doi:10.1021/acschemneuro.6b00137. PMID 27421060.
- ↑ "2015. ÉVI EURÓPAI KÁBÍTÓSZER - JELENTÉS - A MONITOROZÁS 20 ÉVE" (PDF). Hungarian National Focal Point (NFP). June 2015. Retrieved 23 July 2015.
- ↑ "5-fluoro-3,5-AB-PFUPPYCA". Cayman Chemical Company. Retrieved 11 February 2016.
This article is issued from Wikipedia - version of the 8/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.