Nicotinic acetylcholine receptor
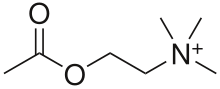
Nicotinic acetylcholine receptors, or nAChRs, are neuron receptor proteins that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs, including the nicotinic receptor agonist nicotine. They are found in the central nervous system of humans, and also play two important roles in the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. Nicotinic receptors are also found in other creatures. In insects, the cholinergic system is limited to the central nervous system.[1]
The nicotinic receptors are considered cholinergic receptors, since they respond to acetylcholine. Nicotinic receptors get their name from nicotine, which does not stimulate the muscarinic acetylcholine receptor, but instead selectively binds to the nicotinic receptor.[2][3][4] The muscarinic acetylcholine receptor likewise gets its name from a chemical that selectively attaches to that receptor -- muscarine. Acetylcholine itself binds to both muscarinic and nicotinic acetylcholine receptors.
As ionotropic receptors, nAChRs are directly linked to ion channels and do not use second messengers (as metabotropic receptors do). Nicotinic acetylcholine receptors are the best-studied of the ionotropic receptors.[2]
Since nicotinic receptors help transmit outgoing signals for the sympathetic and parasympathetic systems, nicotinic receptor antagonists such as hexamethonium interfere with the transmission of these signals. Thus, for example, nicotinic receptor antagonists interfere with the baroreflex that normally corrects changes in blood pressure by sympathetic and parasympathetic stimulation of the heart.
Structure

Nicotinic receptors, with a molecular mass of 290 kDa,[5] are made up of five subunits, arranged symmetrically around a central pore.[2] Each subunit comprises four transmembrane domains with both the N- and C-terminus located extracellularly. They possess similarities with GABAA receptors, glycine receptors, and the type 3 serotonin receptors (which are all ionotropic receptors), or the signature Cys-loop proteins.[6]
In vertebrates, nicotinic receptors are broadly classified into two subtypes based on their primary sites of expression: muscle-type nicotinic receptors and neuronal-type nicotinic receptors. In the muscle-type receptors, found at the neuromuscular junction, receptors are either the embryonic form, composed of α1, β1, γ, and δ subunits in a 2:1:1:1 ratio, or the adult form composed of α1, β1, δ, and ε subunits in a 2:1:1:1 ratio.[2][3][4][7] The neuronal subtypes are various homomeric or heteromeric combinations of twelve different nicotinic receptor subunits: α2−α10 and β2−β4. Examples of the neuronal subtypes include: (α4)3(β2)2, (α4)2(β2)3, and (α7)5. In both muscle-type and neuronal-type receptors, the subunits are somewhat similar to one another, especially in the hydrophobic regions.
Binding the channel
As with all ligand-gated ion channels, opening of the nAChR channel pore requires the binding of a chemical messenger. Several different terms are used to refer to the molecules that bind receptors, such as ligand. As well as the endogenous agonist acetylcholine, agonists of the nAChR are nicotine, epibatidine, and choline. Nicotinic antagonists that block the receptor include hexamethonium.
In muscle-type nAChRs, the acetylcholine binding sites are located at the α and either ε or δ subunits interface (or between two α subunits in the case of homomeric receptors) in the extracellular domain near the N terminus.[3][8] When an agonist binds to the site, all present subunits undergo a conformational change and the channel is opened[9] and a pore with a diameter of about 0.65 nm opens.[3]
Opening the channel
Nicotinic AChRs may exist in different interconvertible conformational states. Binding of an agonist stabilises the open and desensitised states. Opening of the channel allows positively charged ions to move across it; in particular, sodium enters the cell and potassium exits. The net flow of positively charged ions is inward.
The nAChR is a non-selective cation channel, meaning that several different positively charged ions can cross through.[2] It is permeable to Na+ and K+, with some subunit combinations that are also permeable to Ca2+.[3][10][11] The amount of sodium and potassium the channels allow through their pores (their conductance) varies from 50–110 pS, with the conductance depending on the specific subunit composition as well as the permeant ion.[12]
It is interesting to note that, because some neuronal nAChRs are permeable to Ca2+, they can affect the release of other neurotransmitters.[4] The channel usually opens rapidly and tends to remain open until the agonist diffuses away, which usually takes about 1 millisecond.[3] However, AChRs can sometimes open with only one agonist bound and, in rare cases, with no agonist bound, and they can close spontaneously even when ACh is bound. Therefore, ACh binding creates only a probability of pore opening, which increases as more ACh binds.[9]
The nAChR is unable to bind ACh when bound to any of the snake venom α-neurotoxins. These α-neurotoxins antagonistically bind tightly and noncovalently to nAChRs of skeletal muscles, thereby blocking the action of ACh at the postsynaptic membrane, inhibiting ion flow and leading to paralysis and death. The nAChR contains two binding sites for snake venom neurotoxins. Progress towards discovering the dynamics of binding action of these sites has proved difficult, although recent studies using normal mode dynamics[13] have aided in predicting the nature of both the binding mechanisms of snake toxins and of ACh to nAChRs. These studies have shown that a twist-like motion caused by ACh binding is likely responsible for pore opening, and that one or two molecules of α-bungarotoxin (or other long-chain α-neurotoxin) suffice to halt this motion. The toxins seem to lock together neighboring receptor subunits, inhibiting the twist and therefore, the opening motion.[14]
Effects
The activation of receptors by nicotine modifies the state of neurons through two main mechanisms. On one hand, the movement of cations causes a depolarization of the plasma membrane (which results in an excitatory postsynaptic potential in neurons), but also by the activation of voltage-gated ion channels. On the other hand, the entry of calcium acts, either directly or indirectly, on different intracellular cascades. This leads, for example, to the regulation of the activity of some genes or the release of neurotransmitters.
Receptor regulation
Receptor desensitisation
Ligand-bound desensitisation of receptors was first characterised by Katz and Thesleff in the nicotinic acetylcholine receptor.[15]
Prolonged or repeat exposure to a stimulus often results in decreased responsiveness of that receptor toward a stimulus, termed desensitisation. nAChR function can be modulated by phosphorylation[16] by the activation of second messenger-dependent protein kinases. PKA[15] and PKC[17] have been shown to phosphorylate the nAChR resulting in its desensitisation. It has been reported that, after prolonged receptor exposure to the agonist, the agonist itself causes an agonist-induced conformational change in the receptor, resulting in receptor desensitisation.[18] Desensitised receptors can revert to a prolonged open state when an agonist is bound in the presence of a positive allosteric modulator, for example PNU-120596.[19] Also, there is evidence that indicates specific chaperone molecules has regulatory effects on these receptors.[20]
Roles
The subunits of the nicotinic receptors belong to a multigene family (16 members in humans) and the assembly of combinations of subunits results in a large number of different receptors (for more information see the Ligand-Gated Ion Channel database). These receptors, with highly variable kinetic, electrophysiological and pharmacological properties, respond to nicotine differently, at very different effective concentrations. This functional diversity allows them to take part in two major types of neurotransmission. Classical synaptic transmission (wiring transmission) involves the release of high concentrations of neurotransmitter, acting on immediately neighboring receptors. In contrast, paracrine transmission (volume transmission) involves neurotransmitters released by synaptic boutons, which then diffuse through the extra-cellular medium until they reach their receptors, which may be distant. Nicotinic receptors can also be found in different synaptic locations; for example the muscle nicotinic receptor always functions post-synaptically. The neuronal forms of the receptor can be found both post-synaptically (involved in classical neurotransmission) and pre-synaptically[21] where they can influence the release of multiple neurotransmitters.
Subunits
17 vertebrate nAChR subunits have been identified, which are divided into muscle-type and neuronal-type subunits. However, although an α8 subunit/gene is present in avian species such as the chicken, it is not present in human or mammalian species.[22]
The nAChR subunits have been divided into 4 subfamilies (I-IV) based on similarities in protein sequence.[23] In addition, subfamily III has been further divided into 3 tribes.
| Neuronal-type | Muscle-type | ||||
| I | II | III | IV | ||
|---|---|---|---|---|---|
| α9, α10 | α7, α8 | 1 | 2 | 3 | α1, β1, δ, γ, ε |
| α2, α3, α4, α6 | β2, β4 | β3, α5 | |||
- α genes: CHRNA1 (muscle), CHRNA2 (neuronal), CHRNA3, CHRNA4, CHRNA5, CHRNA6, CHRNA7, CHRNA8, CHRNA9, CHRNA10
- β genes: CHRNB1 (muscle), CHRNB2 (neuronal), CHRNB3, CHRNB4
- Other genes: CHRND (delta), CHRNE (epsilon), CHRNG (gamma)
Notable variations
Nicotinic receptors are pentamers of these subunits; i.e., each receptor contains five subunits. Thus, there is an immense potential of variation of the aforementioned subunits. However, some of them are more notable than others, to be specific, (α1)2β1δε (muscle-type), (α3)2(β4)3 (ganglion-type), (α4)2(β2)3 (CNS-type) and (α7)5 (another CNS-type).[24] A comparison follows:
| Receptor-type | Location | Effect; functions | Nicotinic agonists | Nicotinic antagonists |
|---|---|---|---|---|
| Muscle-type: (α1)2β1δε[24] or (α1)2β1δγ |
Neuromuscular junction | EPSP, mainly by increased Na+ and K+ permeability | ||
| Ganglion-type: (α3)2(β4)3 |
autonomic ganglia | EPSP, mainly by increased Na+ and K+ permeability | ||
| Heteromeric CNS-type: (α4)2(β2)3 |
Brain | Post- and presynaptic excitation,[24] mainly by increased Na+ and K+ permeability. Major subtype involved in the attention-enhancing and rewarding effects of nicotine as well as the pathophysiology of nicotine addiction.[26][27][28] |
| |
| Further CNS-type: (α3)2(β4)3 |
Brain | Post- and presynaptic excitation | ||
| Homomeric CNS-type: (α7)5 |
Brain | Post- and presynaptic excitation,[24] mainly by increased Na+, K+ and Ca2+ permeability. Major subtype involved in some of the cognitive effects of nicotine.[29] Moreover, activation of (α7)5 could improve neurovascular coupling response in neurodegenerative disease.[30]
Also involved in the pro-angiogenic effects of nicotine and accelerate the progression of chronic kidney disease in smokers.[31][32][33] |
||
See also
References
- ↑ Yamamoto, Izuru (1999). "Nicotine to Nicotinoids: 1962 to 1997". In Yamamoto, Izuru; Casida, John. Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 3–27
- 1 2 3 4 5 6 7 8 9 10 11 Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience (4th ed.). Sinauer Associates. pp. 122–6. ISBN 978-0-87893-697-7.
- 1 2 3 4 5 6 Siegel G.J.; Agranoff B.W.; Fisher S.K.; Albers R.W. & Uhler M.D. (1999). "Basic Neurochemistry: Molecular, Cellular and Medical Aspects". GABA Receptor Physiology and Pharmacology (6th ed.). American Society for Neurochemistry. Retrieved 2008-10-01.
- 1 2 3 Itier V, Bertrand D (August 2001). "Neuronal nicotinic receptors: from protein structure to function". FEBS Letters. 504 (3): 118–25. doi:10.1016/S0014-5793(01)02702-8. PMID 11532443.
- ↑ Unwin N. (March 4, 2005). "Refined structure of the nicotinic acetylcholine receptor at 4A resolution". Journal of Molecular Biology. 346 (4): 967–89. doi:10.1016/j.jmb.2004.12.031. PMID 15701510.
- ↑ Cascio, M. (May 7, 2004). "Structure and function of the glycine receptor and related nicotinicoid receptors". Journal of Biological Chemistry. 279 (19): 19383–6. doi:10.1074/jbc.R300035200. PMID 15023997.
- ↑ Giniatullin R, Nistri A, Yakel JL (July 2005). "In muscle, the acetylcholine ligand binds to two regions, one region is between the alpha and delta subunits and the other, between the alpha and gamma subunits. Desensitisation of nicotinic ACh receptors: shaping cholinergic signaling". Trends Neurosci. 28 (7): 371–8. doi:10.1016/j.tins.2005.04.009. PMID 15979501.
- ↑ Squire, Larry (2003). Fundamental neuroscience (2nd ed.). Amsterdam: Acad. Press. p. 1426. ISBN 978-0-12-660303-3.
- 1 2 Colquhoun D, Sivilotti LG (June 2004). "Function and structure in glycine receptors and some of their relatives". Trends Neurosci. 27 (6): 337–44. doi:10.1016/j.tins.2004.04.010. PMID 15165738.
- ↑ Beker F, Weber M, Fink RH, Adams DJ (September 2003). "Muscarinic and nicotinic ACh receptor activation differentially mobilize Ca2+ in rat intracardiac ganglion neurons". J. Neurophysiol. 90 (3): 1956–64. doi:10.1152/jn.01079.2002. PMID 12761283.
- ↑ Weber M, Motin L, Gaul S, Beker F, Fink RH, Adams DJ (January 2005). "Intravenous anaesthetics inhibit nicotinic acetylcholine receptor-mediated currents and Ca2+ transients in rat intracardiac ganglion neurons". Br. J. Pharmacol. 144 (1): 98–107. doi:10.1038/sj.bjp.0705942. PMC 1575970
 . PMID 15644873.
. PMID 15644873. - ↑ Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B (22–28 May 1986). "Molecular distinction between fetal and adult forms of muscle acetylcholine receptor". Nature. 321 (6068): 406–11. doi:10.1038/321406a0. PMID 2423878.
- ↑ Levitt, M.; Sander, C.; Stern, P. S. (1985). "Protein normal-mode dynamics: Trypsin inhibitor, crambin, ribonuclease and lysozyme". Journal of Molecular Biology. 181 (3): 423–447. doi:10.1016/0022-2836(85)90230-X. PMID 2580101.
- ↑ Samson, A. O.; Levitt, M. (2008). "Inhibition Mechanism of the Acetylcholine Receptor by α-Neurotoxins as Revealed by Normal-Mode Dynamics". Biochemistry. 47 (13): 4065–4070. doi:10.1021/bi702272j. PMC 2750825
 . PMID 18327915.
. PMID 18327915. - 1 2 Pitchford S, Day JW, Gordon A, Mochly-Rosen D (November 1992). "Nicotinic acetylcholine receptor desensitisation is regulated by activation-induced extracellular adenosine accumulation". Journal of Neuroscience. 12 (11): 4540–4. PMID 1331363.
- ↑ Huganir RL, Greengard P (February 1983). "cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor". Proceedings of the National Academy of Sciences of the United States of America. 80 (4): 1130–4. doi:10.1073/pnas.80.4.1130. PMC 393542
 . PMID 6302672.
. PMID 6302672. - ↑ Safran A, Sagi-Eisenberg R, Neumann D, Fuchs S (August 1987). "Phosphorylation of the acetylcholine receptor by protein kinase C and identification of the phosphorylation site within the receptor delta subunit". The Journal of Biological Chemistry. 262 (22): 10506–10. PMID 3038884.
- ↑ Barrantes FJ (September 1978). "Agonist-mediated changes of the acetylcholine receptor in its membrane environment". Journal of Molecular Biology. 124 (1): 1–26. doi:10.1016/0022-2836(78)90144-4. PMID 712829.
- ↑ Hurst, RS; Hajós, M; Raggenbass, M; Wall, TM; Higdon, NR; Lawson, JA; Rutherford-Root, KL; Berkenpas, MB; Hoffmann, WE; Piotrowski, DW; Groppi, VE; Allaman, G; Ogier, R; Bertrand, S; Bertrand, D; Arneric, SP (April 2005). "A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization". Journal of Neuroscience. 25 (17): 4396–405. doi:10.1523/JNEUROSCI.5269-04.2005. PMID 15858066.
- ↑ Sadigh-Eteghad S, Majdi A, Talebi M, Mahmoudi J, Babri S (2015). "Regulation of nicotinic acetylcholine receptors in Alzheimer׳s disease: A possible role of chaperones". European Journal of Pharmacology. 755: 34–41. doi:10.1016/j.ejphar.2015.02.047. PMID 25771456.
- ↑ Wonnacott S (February 1997). "Presynaptic nicotinic ACh receptors". Trends in Neurosciences. 20 (2): 92–8. doi:10.1016/S0166-2236(96)10073-4. PMID 9023878.
- ↑ Graham A, Court JA, Martin-Ruiz CM, Jaros E, Perry R, Volsen SG, Bose S, Evans N, Ince P, Kuryatov A, Lindstrom J, Gotti C, Perry EK (2002). "Immunohistochemical localisation of nicotinic acetylcholine receptor subunits in human cerebellum". Neuroscience. 113 (3): 493–507. doi:10.1016/S0306-4522(02)00223-3. PMID 12150770.
- ↑ Le Novère N, Changeux JP (February 1995). "Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells". Journal of Molecular Evolution. 40 (2): 155–72. doi:10.1007/BF00167110. PMID 7699721.
- 1 2 3 4 Rang, H. P. (2003). Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4.
- 1 2 Neurosci.pharm - MBC 3320 Acetylcholine
- ↑ Sarter M (August 2015). "Behavioral-cognitive targets for cholinergic enhancement". Current Opinion in Behavioral Sciences. 4: 22–26. doi:10.1016/j.cobeha.2015.01.004.
- ↑ Wu, J; Gao, M; Shen, JX; Shi, WX; Oster, AM; Gutkin, BS (October 2013). "Cortical control of VTA function and influence on nicotine reward.". Biochemical Pharmacology. 86 (8): 1173–80. doi:10.1016/j.bcp.2013.07.013. PMID 23933294.
- ↑ "Nicotine: Biological activity". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. Retrieved 7 February 2016.
Kis as follows; α2β4=9900nM [5], α3β2=14nM [1], α3β4=187nM [1], α4β2=1nM [4,6]. Due to the heterogeneity of nACh channels we have not tagged a primary drug target for nicotine, although the α4β2 is reported to be the predominant high affinity subtype in the brain which mediates nicotine addiction [2-3].
- ↑ Levin, ED (May 2012). "α7-Nicotinic receptors and cognition.". Current Drug Targets. 13 (5): 602–6. doi:10.2174/138945012800398937. PMID 22300026.
- ↑ Sadigh-Eteghad S, Mahmoudi J, Babri S, Talebi M (2015). "Effect of alpha-7 nicotinic acetylcholine receptor activation on beta-amyloid induced recognition memory impairment. Possible role of neurovascular function.". Acta Cirurgica Brasileira. 30 (11): 736–42. doi:10.1590/S0102-865020150110000003. PMID 26647792.
- ↑ Lee, J; Cooke, JP (November 2012). "Nicotine and pathological angiogenesis". Life Sciences. 91 (21–22): 1058–64. doi:10.1016/j.lfs.2012.06.032. PMC 3695741
 . PMID 22796717.
. PMID 22796717. - ↑ Jain, G; Jaimes, EA (October 2013). "Nicotine signaling and progression of chronic kidney disease in smokers.". Biochemical Pharmacology. 86 (8): 1215–23. doi:10.1016/j.bcp.2013.07.014. PMID 23892062.
- ↑ Mihalak KB, Carroll FI, Luetje CW; Carroll; Luetje (2006). "Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors". Mol. Pharmacol. 70 (3): 801–805. doi:10.1124/mol.106.025130. PMID 16766716.
External links
| Wikiversity has learning materials about Poisson–Boltzmann profile for an ion channel |