Lacidipine
 | |
| Clinical data | |
|---|---|
| Trade names | Lacipil, Motens |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | C08CA09 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~10% |
| Protein binding | >95% |
| Metabolism | Hepatic |
| Onset of action | 30–50 min |
| Biological half-life | 13–19 hours |
| Excretion | Feces (~70%) |
| Identifiers | |
| |
| CAS Number | 103890-78-4 |
| PubChem (CID) | 5311217 |
| ChemSpider | 4470736 |
| UNII |
260080034N |
| KEGG |
D04657 |
| ECHA InfoCard | 100.166.373 |
| Chemical and physical data | |
| Formula | C26H33NO6 |
| Molar mass | 455.543 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Lacidipine (tradenames Lacipil (GSK) or Motens (Boehringer Ingelheim)) is a calcium channel blocker. It is available as tablets containing 2 or 4 mg.
Synthesis
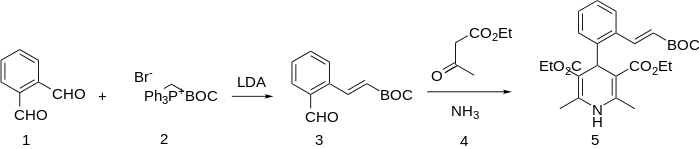
The synthesis starts with the Wittig reaction of phthalaldehyde (1) with the ylide from triphenylphosphonium salt (2). The trans stereochemistry of the product (3) follows from the fact that the rxn is not carried out under salt-free conditions; selectivity for monoalkylation is probably due to steric hindrance from the newly introduced adjacent side chain to the adjacent formyl group. reaction of tat intermediate with ethyl acetoacetate and ammonia gives the dihydropyridine lacidipine (5).
Notes
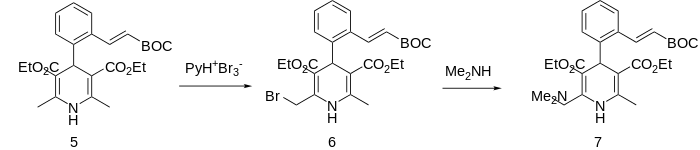
Further modification of this compound depends on the allylic nature of the ring methyl groups. Thus reaction of 5 with pyridinium perbromide leads to the bromination of one groups and the formation of 6. The displacement of halogen by dimethylamine leads to the tertiary amine teludipine (7).