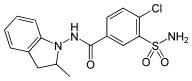
Indapamide
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684062 |
| Pregnancy category |
|
| Routes of administration | Oral tablet |
| ATC code | C03BA11 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 71–79% |
| Metabolism | Hepatic |
| Biological half-life | 14–18 hours |
| Identifiers | |
| |
| CAS Number |
26807-65-8 |
| PubChem (CID) | 3702 |
| IUPHAR/BPS | 7203 |
| DrugBank |
DB00808 |
| ChemSpider |
3574 |
| UNII |
F089I0511L |
| KEGG |
D00345 |
| ChEMBL |
CHEMBL406 |
| ECHA InfoCard | 100.043.633 |
| Chemical and physical data | |
| Formula | C16H16ClN3O3S |
| Molar mass | 365.835 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Indapamide is a thiazide-like diuretic[1] drug marketed by Servier, generally used in the treatment of hypertension, as well as decompensated heart failure. Combination preparations with perindopril (an ACE inhibitor antihypertensive) are also available.
Form and composition
Indapamide is available generically as 1.25 mg and 2.5 mg non-scored tablets.[2] It is now also available in SR (sustained release) form.
Indications
Hypertension and edema due to congestive heart failure. Indapamide has been proven in the HYVET trial to reduce stroke and all cause mortality when given with or without perindopril to people over the age of 80 for the treatment of hypertension.[3]
Dosage and administration
The adult dosage is 1.25 to 5 mg, orally and once daily, usually in the morning.
Contraindications
Indapamide is contraindicated in known hypersensitivity to sulfonamides, severe kidney failure, hepatic encephalopathy or severe liver failure, and a low blood potassium level.
There is insufficient safety data to recommend indapamide use in pregnancy or breastfeeding.
Interactions
Caution is advised in the combination of indapamide with lithium and nonantiarrhythmic drugs causing wave-burst arrhythmia (astemizole, bepridil, IV erythromycin, halofantrine, pentamidine, sultopride, terfenadine, vincamine).
Precautions
Monitoring of potassium and uric acid serum levels is recommended, especially in subjects with a predisposition or a sensitivity to hypokalemia and in patients with gout.
Adverse effects
Commonly reported adverse events are hypokalemia (low potassium levels), fatigue, orthostatic hypotension (blood pressure decrease on standing up) and allergic manifestations.
Overdosage
Symptoms of overdosage would be those associated with a diuretic effect, i.e. electrolyte disturbances, hypotension, and muscular weakness. Treatment should be symptomatic, directed at correcting electrolyte abnormalities.
See also
References
- ↑ Indapamide at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ "Lexicomp Online Login". lexi.com.
- ↑ "HYVET Trial".