Alfatradiol
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | 17-Epiestradiol |
| CAS Number |
57-91-0 |
| PubChem (CID) | 68570 |
| ChemSpider |
61840 |
| UNII |
3VQ38D63M7 |
| ChEBI |
CHEBI:17160 |
| ChEMBL |
CHEMBL286452 |
| Chemical and physical data | |
| Formula | C18H24O2 |
| Molar mass | 272.37 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Alfatradiol (INN) (brand names Ell-Cranell Alpha, Pantostin in Germany),[1] also known as 17α-estradiol or as 17-epiestradiol, is a steroidal weak estrogen and potent 5α-reductase inhibitor used topically in the treatment of androgenic alopecia (hair loss) in men and women.[2] It is a stereoisomer of the endogenous steroid hormone 17β-estradiol.
Uses
Alfatradiol is used in form of an ethanolic solution for topical application on the scalp. Similarly to other drugs against alopecia, topical or oral, it has to be applied continuously to prevent further hair loss.[3] Regrowth of hair that was already lost is only possible to a limited extent. In general, advanced alopecia does not respond well to medical treatment, which has historically been thought to be a consequence of the hair roots being lost.[4]
A university-led study (including several authors who are advisors to companies such as Pfizer) in 103 women comparing alfatradiol to minoxidil, another topical hair loss treatment, found the latter to be more effective. In contrast to minoxidil, alfatradiol did not result in an increase of hair density or thickness, but only in slowing down or stabilization of hair loss in this study.[5] In an earlier study, no systemic side effects were noted, and 17α-estradiol was found to reduce androgenic hair loss, though it was not effective at growing new hair.[6]
Contraindications
Nothing is known about the use of alfatradiol during pregnancy or lactation, or in patients under 18 years of age. The package leaflet recommends against using it under these circumstances.[7]
Adverse effects
Local burning or itching is not an effect of alfatradiol, but of the ethanol in the solvent. The solution can stimulate sebum production.[7]
Chemistry and pharmacology
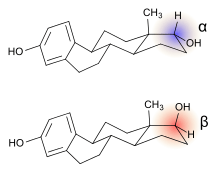
Alfatradiol (17α-estradiol) is distinguished from estradiol (17β-estradiol), the predominant sex hormone in females, only by the stereochemistry of the carbon atom 17. In contrast to 17β-estradiol, 17α-estradiol, while it still binds to the estrogen receptor, has less or no feminizing estrogen activity depending on its dosage and the tissue it is affecting.[8] Alfatradiol acts as an inhibitor of the enzyme 5α-reductase, which is responsible for the activation of testosterone to dihydrotestosterone, and which plays a role in regulating hair growth.[3] 17α-Estradiol has been studied as a therapeutic with potential to treat Alzheimer's and Parkinson's disease and other patients suffering from neurodegenerative diseases.[9] 17α-Estradiol (as the sodium salt of its sulfated form) is a minor component (<10%) of hormone replacement products (such as Premarin), which have been studied and/or marketed in women and men since the 1930s. A survey of the effects of various forms of 17α-estradiol in humans on biochemical parameters, efficacy, estrogenicity, metabolism, safety, and tolerability has been published.[10]
Alfatradiol binds to the ERα and ERβ with 58% and 11% of the relative binding affinity of 17β-estradiol.[11] However, it has 100-fold lower estrogenic activity relative to estradiol,[12] which may be due to differences in the intrinsic activity of the two compounds. On the other hand, alfatradiol has been found to bind to and activate the brain-expressed ER-X with a greater potency than estradiol, indicating that it may be the predominant endogenous ligand for the receptor.[13]
Research
17α-Estradiol improved metabolic function, reduced insulin resistance, decreased intra-abdominal fat, and decreased inflammation in old male mice without inducing feminization, suggesting potential future use for type 2 diabetes. [14]
See also
- Alfatradiol/dexamethasone (Ell-Cranell dexa)
- Estradiol 17alpha-dehydrogenase
- Finasteride
- Ketoconazole
- Minoxidil
- 17α-Epiestriol
- Epimestrol
References
- ↑ "Recommended International Nonproprietary Names (rec. Inn): List 46". WHO Drug Information. 15 (3&4). 2001.
- ↑ Berger, Artur; Wachter, Helmut, eds. (1998). Hunnius Pharmazeutisches Wörterbuch (in German) (8 ed.). Walter de Gruyter Verlag. p. 486. ISBN 3-11-015793-4.
- 1 2 Mutschler, Ernst; Gerd Geisslinger; Heyo K. Kroemer; Monika Schäfer-Korting (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 453. ISBN 3-8047-1763-2.
- ↑ Jasek, W, ed. (2007). Austria-Codex (in German). 4 (2007/2008 ed.). Vienna: Österreichischer Apothekerverlag. p. 9673. ISBN 978-3-85200-181-4.
- ↑ Blume-Peytavi, U.; Kunte, C.; Krisp, A.; Bartels, N. G.; Ellwanger, U.; Hoffmann, R. (2007). "Comparison of the efficacy and safety of topical minoxidil and topical alfatradiol in the treatment of androgenetic alopecia in women". JDDG. 5 (5): 391–5. doi:10.1111/j.1610-0387.2007.06295.x. PMID 17451383.
- ↑ C.E. Orfanos, L. Vogels, Local therapy of androgenetic alopecia with 17α-estradiol. A controlled randomized double-blind study, Dermatologica 1980, 161:124-132.
- 1 2 Dootz, Hildegard (ed.). Rote Liste (in German) (2005 ed.). Aulendorf: Editio Cantor Verlag. 32 369. ISBN 3-87193-306-6.
- ↑ W.H. Moos, J.A. Dykens, N. Howell, 17α-Estradiol: A Less-Feminizing Estrogen, Drug Dev. Res. 2008, 69:177-184.
- ↑ J.A. Dykens, W.H. Moos, N. Howell, Development of 17α-Estradiol as a Neuroprotective Therapeutic Agent. Rationale and Results from a Phase I Clinical Study, Ann. N. Y. Acad. Sci. 2005, 1052:116-135.
- ↑ W.H. Moos, J.A. Dykens, D. Nohynek, E. Rubinchik, N. Howell, Review of the Effects of 17α-Estradiol in Humans: A Less Feminizing Estrogen with Neuroprotective Potential, Drug Dev. Res. 2009, 70:1-21.
- ↑ Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ↑ Ralph M. Trüeb; Won-Soo Lee (13 February 2014). Male Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 93–. ISBN 978-3-319-03233-7.
- ↑ Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS (2005). "17alpha-estradiol: a brain-active estrogen?". Endocrinology. 146 (9): 3843–50. doi:10.1210/en.2004-1616. PMID 15947006.
- ↑ Stout, MB; Steyn, FJ; Jurczak, MJ; Camporez, JG; Zhu, Y; Hawse, JR; Jurk, D; Palmer, AK; Xu, M; Pirtskhalava, T; Evans, GL; de Souza Santos, R; Frank, AP; White, TA; Monroe, DG; Singh, RJ; Casaclang-Verzosa, G; Miller, JD; Clegg, DJ; LeBrasseur, NK; von Zglinicki, T; Shulman, GI; Tchkonia, T; Kirkland, JL (Jan 24, 2016). "17α-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization". J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glv309. PMID 26809497.