Trifluoromethylation
Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound.[1][2][3][4] Trifluoromethylated compounds are of some importance in pharma and agrochemicals. Several notable pharmaceutical compounds have a trifluoromethyl group incorporated: fluoxetine, mefloquine, Leflunomide, nulitamide, dutasteride, bicalutamide, aprepitant, celecoxib, fipronil, fluazinam, penthiopyrad, picoxystrobin, fluridone, norflurazon, sorafenib and triflurazin. A relevant agrochemical is trifluralin The development of synthetic methods for adding trifluoromethyl groups to chemical compounds is actively pursued in academic research.
History
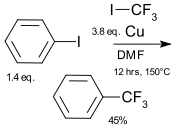
The first to investigate trifluoromethyl groups in relationship to biological activity was F. Lehmann in 1927.[5] An early review appeared in 1958.[6] An early synthetic method was developed by Frédéric Swarts in 1892,[7] based on antimony fluoride. In this reaction benzotrichloride was reacted with SbF3 to form PhCF2Cl and PhCF3. In the 1930s Kinetic Chemicals and IG Farben replaced SbF3 with HF. The McLoughlin-Thrower reaction (1968) is an early coupling reaction using iodofluoroalkanes, iodoaromatic compounds and copper.[8] In 1969 Kobayashi & Kumadaki adapted their protocol for trifluoromethylations.[9][10]
 |
| McLoughlin-Thrower reaction (1968) |
Reagents
Trifluoromethyltrimethylsilane
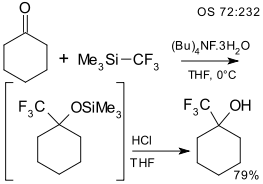
Preparation of the Trifluoromethyltrimethylsilane was reported by Ingo Ruppert in 1984.[11] In 1989, Prakash and Olah first reported activation of TMSCF3 by fluoride to perform nucleophilic trifluoromethylation of carbonyl compounds.[12] In the same year, Stahly described similar reactions for the synthesis of trifluoromethylated phenols and anilines.[13] Since then TMSCF3 has been widely used as a nucleophilic trifluoromethylating agent.[14][15]
An example is the trifluoromethylation of cyclohexanone in THF using tetrabutylammonium fluoride.[16]
 |
| Trifluoromethylation using
Trifluoromethyltrimethylsilane[16] |
The substrates can be aryl halides.[17][18] Potassium (trifluoromethyl)trimethoxyborate for this purpose has been synthesised from B(OMe)3, CF3SiMe3, and KF.[19] Aryl functionalization via C-H activation has also been reported[20][21]
Sodium trifluoroacetate
Sodium trifluoroacetate as a reagent for trifluoromethylations was introduced by Matsui in 1981. In the original scope the substrate was an aromatic halide and the metal salt copper(I)iodide.[22][23]
Trifluoromethane
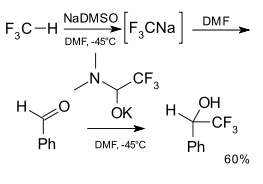
Fluoroform (CF3H) has been employed as a trifluoromethylation reagent for aldehydes in combination with a strong base[24]
 |
| Trifluoromethylation fluoroform folleas 1998[24] |
Trifluoroiodomethane
Trifluoroiodomethane is a reagent in aromatic coupling reactions. It has also been used with enones, for example with chalcone, a reaction catalysed by diethyl zinc and Wilkinson's catalyst:[25]
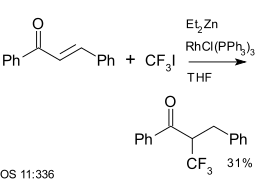 |
| Trifluoromethylation using diethyl zinc and Wilkinson's catalyst[25] |
Trifluoromethyl Sulfone
Trifluoromethyl Sulfone (PhSO2CF3) and Trifluoromethyl Sulfoxide (PhSOCF3) can be used for trifluoromethylations of electrophiles[26]
Trifluoromethanesulfonyl chloride
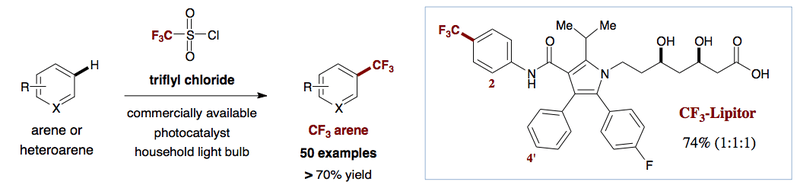
Trifluoromethanesulfonyl chloride (or Triflyl Chloride, CF3SO2Cl) can be used in a highly efficient method to introduce a trifluoromethyl group to aromatic and heteroaromatic systems, including known pharmaceuticals such as Lipitor. The chemistry is general and mild, and uses a photoredox catalyst and a light source at room temperature.[27]
Sodium trifluoromethanesulfinate
Sodium trifluoromethanesulfinate (CF3SO2Na) as a trifluoromethylation reagent was introduced by Langlois in 1991.[28] The reaction requires t-butyl hydroperoxide and generally a metal and proceeds through a radical mechanism. The reagent has been applied with heterocyclic substrates[29]
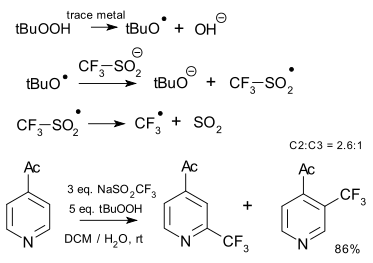 |
| Trifluorination Langlois reagent 2011[29] |
Umemoto reagents
Umemoto reagents are (trifluoromethyl)dibenzoheterocyclic salts.[30][31]
Trifluoromethyl-metal reagents
Many CF3-containing metal complexes have been prepared, and some are useful for trifluoromethylation. The most obvious reagent is CF3Li, which can be generated by lithium-iodide exchange. This compound is however unstable even at low temperatures. It degrades to lithium fluoride and difluorocarbene. Trifluoromethyl copper(I) reagents are more useful. These reagents are generated in situ by reaction of CF3I with copper powder in polar solvents.[32] Hg(CF3)2, prepared by decarboxylation of the trifluoroacetate, has proven useful for the trifluoromethylation of other metals.[33]
Reaction types
Aromatic coupling reactions
In coupling reactions between aromatic compounds and metal-trifluoromethyl complexes the metal is usually copper, Pd and Ni are less prominent.[1] The reactions are stoichiometric or catalytic. In the McLoughlin-Thrower reaction (1962) iodobenzene reacts with trifluoroiodomethane (CF3I) and copper powder in dimethylformamide at 150 °C to trifluoromethylbenzene. The intermediate in this reaction type is a perfluoromethyl-metal complex.
A palladium acetate catalysed reaction described in 1982 used zinc powder with the main intermediate believed to be CF3ZnI with Pd(0) is the active catalyst.[34][35] The first copper catalysed coupling was reported in 2009 and based on an iodoarene, a trifluoromethylsilane, copper iodide and 1,10-phenanthroline.[36] Variations include another CF3 donor potassium (trifluoromethyl)trimethoxyborate,[37] the use of aryl boronic acids[38][39] or the use of a trifluoromethyl sulfonium salt[40] or the use of a trifluoromethylcopper(I) phenanthroline complex.[41] A catalytic palladium catalysed reaction was reported in 2010 using aryl halides, (trifluoromethyl)triethylsilane and allylpalladium chloride dimer[42]
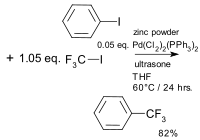 |
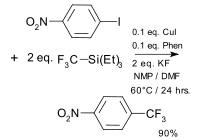 |
| Aromatic trifluoromethylation Kitazume 1982[34] | Aromatic catalytic
trifluoromethylation Oishi 2009[36] |
Radical trifluoromethylation
In radical trifluoromethylation the active species is the trifluoromethyl free radical.[43] Reagents such as bromotrifluoromethane and haloform have been used for this purpose[44][45][46] but in response to the Montreal Protocol alternatives such as trifluoroiodomethane have been developed as replacement.[47][48] One particular combination is CF3I / triethylborane[49][50] Other reagents that generate the CF3 radical are sodium trifluoromethanesulfinate and bis(trifluoroacetyl) peroxide.
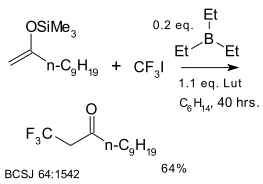 |
| Trifluoromethylation using CF3I and triethylborane.
The base is 2,6-lutidine[49] |
In the CF3 radical the fluorine atom is an electron-withdrawing group via the inductive effect but also a weak pi donor through interaction of the fluorine lone pair with the radical center's SOMO. Compared to the methyl radical the CF3 radical is pyramidal (angle 107.8 °C ) with a large inversion barrier, electrophilic and also more reactive. In reaction with styrene it is 440 times more reactive.[51] An early report (1949) describes the photochemical reaction of iodotrifluoromethane with ethylene to 3-iodo-1,1,1-trifluoropropane.[52] Reagents that have been reported for the direct trifluoromethylation of arenes are CF3I, CF3Br (thermal or photochemical), silver trifluoroacetate/TiO2 (photchemical) and sodium trifluoromethanesulfinate/Cu(OSO2CF3)2/tBuOOH.
Nucleophilic trifluoromethylation
In nucleophilic trifluoromethylation the active species is the CF3− anion.[53] It was, however, widely believed that the trifluoromethyl anion is a transient species and thus cannot be isolated or observed in the condensed phase. Contrary to the popular belief, the CF3 anion, with [K(18-crown-6)]+ as a countercation, was produced and characterized by Prakash and coworkers.[54] The challenges associated with observation of CF3 anion are alluded to its strong basic nature and its tendency to form pentacoordinated silicon species, such as [Me3Si(CF3)2]− or [Me3Si(F)(CF3)]−.
The reactivity of fluoroform in combination with a strong base such as t-BuOK with carbonyl compounds in DMF is an example.[53] Here CF3− and DMF form an hemiaminolate adduct ([Me2NCH(O)CF3]K).[24][55][56][57]
 |
| trifluoromethylation using methyl fluorosulfonyldifluoroacetate.
The intermediate is CF3Cu[58] |
Electrophilic trifluoromethylation
In electrophilic trifluoromethylation the active trifluoromethyl donor group carries a positive charge.[59][60] Production of an CF3+ cation has been described as "extemely hard" [61] The first relevant reagent, a diaryl(trifluoromethyl) sulfonium salt (Ar2S+CF3SbF6−) was developed in 1984 by reaction of an aryltrifluoromethyl sulfoxide 1 with SF3+SbF6− followed by reaction with an electron-rich arene.[62] The reagent was used in trifluoromethylation of a thiophenolate. S-(trifluoromethyl)dibenzothiophenium tetrafluoroborate is a commercially available and known trifluoromethylation reagent based on the same principle first documented in 1990.[63][64] In this type of compound sulfur has been replaced by oxygen, selenium and tellurium. Examples of substrates that have been investigated are pyridine, aniline, triphenylphosphine and the lithium salt of phenylacetylene.
dibenzothiophenium_trifluoromethanesulfonate.svg.png) |
dibenzothiophenium_tetraborate.svg.png) |
-1%2C2-benziodoxole.svg.png) |
| 5-(Trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate |
5-(Trifluoromethyl)dibenzothiophenium tetraborate | 3,3-Dimethyl-1-(trifluoromethyl)-1,2-benziodoxole |
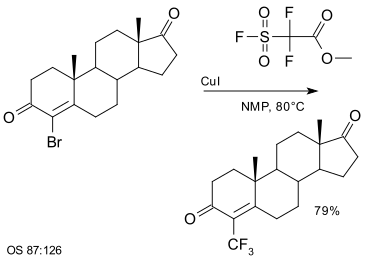
Another group of trifluoromethyl donors are hypervalent iodine(III)–CF3 reagents for example 3,3-dimethyl-1-(trifluoromethyl)-1,2-benziodoxole.[65][66][67][68] Substrates are thiols, alcohols, phosphines, (hetero) arenes,[69] unactivated olefins[70] and unsaturated carboxylic acids.[71]
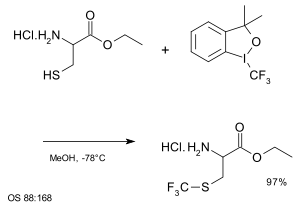 |
| Trifluoromethylation at a thiol group using hypervalent iodine [69] |
The reaction mechanism of electrophilic trifluoromethylations has been described as controversial with polar substitution or single electron transfer as likely candidates [61]
Asymmetric trifluoromethylation
In asymmetric trifluoromethylation the trifluoromethyl group is added to the substrate in an enantioselective way.[72][73] Ruppert's reagent has been used for this purpose in an asymmetric induction approach to functionalise chiral amino acid derivates,[74] carbohydrates,[75] and steroids. Because Ruppert's reagent requires a tetraalkylammonium fluoride, chiral ammonium fluorides have been employed in asymmetric catalysis.[76][77] In the field of electrophilic trifluoromethylation an early contribution involved reaction of a metal enolate with a trifluoromethyl chalcogen salt in presence of a chiral boron catalyst[78]
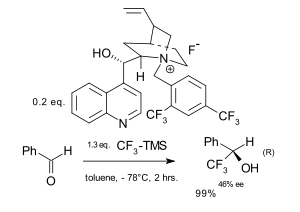 |
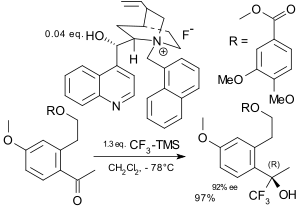 |
| Asymmetic trifluorination Iseki 1994[76] | Asymmetic trifluorination Caron 2003[77] |
More recent examples of highly enantioselective methods for the α-trifluoromethylation of carbonyls are available through enamine catalysis of aldehydes (photoredox[79] or iodonium[80]), copper catalysis of β-ketoesters,[81] and radical addition to zirconium enolates.[82]
References
- 1 2 Tomashenko, O. A.; Grushin, V. V. (2011). "Aromatic Trifluoromethylation with Metal Complexes". Chemical Reviews. 111 (8): 4475–4521. doi:10.1021/cr1004293.
- ↑ Furuya, T.; Kamlet, A. S.; Ritter, T. (2011). "Catalysis for fluorination and trifluoromethylation". Nature. 473 (7348): 470–477. doi:10.1038/nature10108.
- ↑ Besset, T.; Schneider, C. D.; Cahard, D. (2012). "Tamed Arene and Heteroarene Trifluoromethylation". Angewandte Chemie International Edition. 51 (21): 5048–5050. doi:10.1002/anie.201201012.
- ↑ Alonso, C. N.; Martínez De Marigorta, E.; Rubiales, G.; Palacios, F. (2015). "Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes". Chemical Reviews. 115 (4): 1847–1935. doi:10.1021/cr500368h.
- ↑ Lehmann, F. "Chemical constitution and activity. Aromatic fluorine compounds." Arch. exp. Pathol. Pharmakol 130 (1928): 250-255.
- ↑ Yale, H. L. (1959). "The Trifluoromethyl Group in Medical Chemistry". Journal of Medicinal and Pharmaceutical Chemistry. 1 (2): 121–133. doi:10.1021/jm50003a001.
- ↑ Swarts (1892). Acad. Roy. Belg. 3 (24): 474. Missing or empty
|title=(help) - ↑ McLoughlin, V. C. R.; Thrower, J. (1969). "A route to fluoroalkyl-substituted aromatic compounds involving fluoroalkylcopper intermediates". Tetrahedron. 25 (24): 5921–5940. doi:10.1016/S0040-4020(01)83100-8.
- ↑ Kobayashi, Y.; Kumadaki, I. (1969). "Trifluoromethylation of aromatic compounds". Tetrahedron Letters. 10 (47): 4095–4096. doi:10.1016/S0040-4039(01)88624-X.
- ↑ Folléas, B. ̂T.; Marek, I.; Normant, J. F.; Saint-Jalmes, L. (2000). "Fluoroform: An Efficient Precursor for the Trifluoromethylation of Aldehydes". Tetrahedron. 56 (2): 275–283. doi:10.1016/S0040-4020(99)00951-5.
- ↑ Ruppert, Ingo; Schlich, Klaus; Volbach, Wolfgang (January 1984). "Die ersten CF3-substituierten organyl(chlor)silane". Tetrahedron Letters. 25 (21): 2195–2198. doi:10.1016/S0040-4039(01)80208-2.
- ↑ Prakash, G. K. Surya; Krishnamurti, Ramesh; Olah, George A. (January 1989). "Synthetic methods and reactions. 141. Fluoride-induced trifluoromethylation of carbonyl compounds with trifluoromethyltrimethylsilane (TMS-CF3). A trifluoromethide equivalent". Journal of the American Chemical Society. 111 (1): 393–395. doi:10.1021/ja00183a073.
- ↑ Stahly, G. Patrick; Bell, Donald R. (June 1989). "A new method for synthesis of trifluoromethyl-substituted phenols and anilines". The Journal of Organic Chemistry. 54 (12): 2873–2877. doi:10.1021/jo00273a020.
- ↑ G. K. Surya Prakash; Andrei K. Yudin (1997). "Perfluoroalkylation with Organosilicon Reagents". Chem. Rev. 97 (3): 757–86. doi:10.1021/cr9408991. PMID 11848888.
- ↑ Xiao Liu, Cong Xu, Mang Wang, and Qun Liu (2015). "Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond". Chem. Rev. 115 (2): 683–730. doi:10.1021/cr400473a. PMID 24754488.
- 1 2 Ramaiah, Pichika; Krishnamurti, Ramesh; K. Surya Prakash, G. (1995). "1-TRIFLUOROMETHYL-1-CYCLOHEXANOL". Org. Synth. 72 (72): 232. doi:10.15227/orgsyn.072.0232.
- ↑ Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. (2011). "A Broadly Applicable Copper Reagent for Trifluoromethylations and Perfluoroalkylations of Aryl Iodides and Bromides". Angew. Chem. 123 (16): 3877–3882. doi:10.1002/ange.201100633.
- ↑ Oishi, Masahiro; Kondo, Hideaki; Amii, Hideki (2009). "Aromatic trifluoromethylation catalytic in copper". Chem. Commun. 2009 (14): 1909–1911. doi:10.1039/B823249K.
- ↑ Knauber, T.; Arikan, F.; Röschenthaler, G.-V.; Gooßen, L. J. (2011). "Copper-Catalyzed Trifluoromethylation of Aryl Iodides with Potassium (Trifluoromethyl)trimethoxyborate". Chem. Eur. J. 17 (9): 2689–2697. doi:10.1002/chem.201002749. PMID 21274956.
- ↑ Ye, Yingda; Lee, Shin Hee; Sanford, Melanie S. (2011). "Silver-Mediated Trifluoromethylation of Arenes Using TMSCF3". Sanford Organic Letters. 13 (20): 5464–5467. doi:10.1021/ol202174a.
- ↑ Hafner, A.; Bräse, S. (2012). "Ortho-Trifluoromethylation of Functionalized Aromatic Triazenes". Angew. Chem. Int. Ed. 51 (15): 3713–3715. doi:10.1002/anie.201107414.
- ↑ Matsui, Kiyohide; Tobita, Etsuko; Ando, Midori; Kondo, Kiyosi (1981). "A convenient trifluoromethylation of aromatic halides with sodium trifluoroacetate.". Chemistry Letters (12): 1719–1720. doi:10.1246/cl.1981.1719.
- ↑ Langlois, Bernard R.; Roques, Nicolas (October 2007). "Nucleophilic trifluoromethylation of aryl halides with methyl trifluoroacetate". Journal of Fluorine Chemistry. 128 (10): 1318–1325. doi:10.1016/j.jfluchem.2007.08.001.
- 1 2 3 Folléas, Benoît; Marek, Ilan; Normant, Jean-F; Jalmes, Laurent Saint (May 1998). "Fluoroform: an efficient precursor for the trifluoromethylation of aldehydes". Tetrahedron Letters. 39 (19): 2973–2976. doi:10.1016/S0040-4039(98)00391-8.
- 1 2 Sato, Kazuyuki; Omote, Masaaki; Ando, Akira; Kumadaki, Itsumaro (2006). "TRIFLUOROMETHYLATION AT THE a-POSITION OF b,b-UNSATURATED KETONES: 4-PHENYL-3-(TRIFLUOROMETHYL)BUTAN-2-ONE". Org. Synth. 83 (83): 177. doi:10.15227/orgsyn.083.0177.
- ↑ Prakash, G. K. Surya; Hu, Jinbo; Olah, George A. (September 2003). "Alkoxide- and Hydroxide-Induced Nucleophilic Trifluoromethylation Using Trifluoromethyl Sulfone or Sulfoxide". Organic Letters. 5 (18): 3253–3256. doi:10.1021/ol035045u.
- ↑ Nagib, David A.; MacMillan, David W. C. (8 December 2011). "Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis". Nature. 480 (7376): 224–228. doi:10.1038/nature10647.
- ↑ Langlois, Bernard R.; Laurent, Eliane; Roidot, Nathalie (December 1991). "Trifluoromethylation of aromatic compounds with sodium trifluoromethanesulfinate under oxidative conditions.". Tetrahedron Letters. 32 (51): 7525–7528. doi:10.1016/0040-4039(91)80524-A.
- 1 2 Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. (15 August 2011). "Innate C-H trifluoromethylation of heterocycles". Proceedings of the National Academy of Sciences. 108 (35): 14411–14415. doi:10.1073/pnas.1109059108. PMC 3167544
 . PMID 21844378.
. PMID 21844378. - ↑ Zhang, Cai (11 July 2014). "Recent advances in trifluoromethylation of organic compounds using Umemoto's reagents". Organic & Biomolecular Chemistry. 12 (34): 6580. doi:10.1039/C4OB00671B.
- ↑ Li, Huiqin (3 September 2012). "Umemoto's Reagent". Synlett. 23 (15): 2289–2290. doi:10.1055/s-0032-1317176.
- ↑ Donald J. Burton, Long Lu "Fluorinated Organometallic Compounds" Topics in Current Chemistry, 1997, Vol. 193, p. 45.
- ↑ Reint Eujen "Bis(Trifluoromethyl)Mercury" 1986, volume 24, p. 52. doi:10.1002/9780470132555.ch16
- 1 2 Kitazume, Tomoya; Ishikawa, Nobuo (1982). "PALLADIUM-CATALYZED CROSS-COUPLING REACTIONS BETWEEN ALLYL, VINYL OR ARYL HALIDE AND PERFLUOROALKYL IODIDE WITH ZINC AND ULTRASONIC IRRADIATION". Chemistry Letters. 11 (1): 137–140. doi:10.1246/cl.1982.137.
- ↑ Kitazume, Tomoya; Ishikawa, Nobuo (1985). "Ultrasound-promoted selective perfluoroalkylation on the desired position of organic molecules". Journal of the American Chemical Society. 107 (18): 5186–5191. doi:10.1021/ja00304a026.
- 1 2 Oishi, M.; Kondo, H.; Amii, H. (2009). "Aromatic trifluoromethylation catalytic in copper". Chem. Commun. 2009 (14): 1909–1911. doi:10.1039/B823249K.
- ↑ Knauber, T.; Arikan, F.; Roschenthaler, G.-V.; Gooßen, L. J. (2011). "Copper-catalyzed trifluoromethylation of aryl iodides with potassium (trifluoromethyl) trimethoxyborate". Chem. Eur. J. 17 (9): 2689–2697. doi:10.1002/chem.201002749. PMID 21274956.
- ↑ Chu, L.; Qing, F.-L. (2010). "Copper-mediated oxidative trifluoromethylation of boronic acids". Org. Lett. 12 (21): 5060–5063. doi:10.1021/ol1023135. PMID 20923196.
- ↑ Senecal, Todd D.; Parsons, Andrew T.; Buchwald, Stephen L. (18 February 2011). "Room Temperature Aryl Trifluoromethylation via Copper-Mediated Oxidative Cross-Coupling". The Journal of Organic Chemistry. 76 (4): 1174–1176. doi:10.1021/jo1023377.
- ↑ Cheng-, Cheng-Pan; Zhang, Pan; Wang, Ling; Prof, Chun-Tao; Chen, Yun; Zhang, Tao; Gu, Cheng; -Qing-, Chun Dr. Yu-; Ji-Chang, Xiao; et al. (2011). "Copper-mediated trifluoromethylation of heteroaromatic compounds by trifluoromethyl sulfonium salts". Angew. Chem. Int. Ed. 50 (8): 1896–1900. doi:10.1002/anie.201006823.
- ↑ Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. (2011). "A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides". Angew. Chem. Int. Ed. 50 (16): 3793–3798. doi:10.1002/anie.201100633.
- ↑ Cho, E. J.; Senecal, T. D.; Kinzel, T.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. (24 June 2010). "The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides". Science. 328 (5986): 1679–1681. doi:10.1126/science.1190524.
- ↑ Ma, Jun-An; Cahard, Dominique (September 2007). "Strategies for nucleophilic, electrophilic, and radical trifluoromethylations". Journal of Fluorine Chemistry. 128 (9): 975–996. doi:10.1016/j.jfluchem.2007.04.026.
- ↑ Andrieux, Claude P.; Gelis, Laurence; Saveant, Jean-Michel (January 1989). "Unusual reactions resulting from the addition on olefins of trifluoromethyl radicals obtained from dissociative electron transfer between electrochemically generated aromatic anion radicals and trifluoromethyl bromide". Tetrahedron Letters. 30 (37): 4961–4964. doi:10.1016/S0040-4039(01)80554-2.
- ↑ Uneyama, Kenji; Kitagawa, Kouichi; Kitagawa, Kouichi (January 1991). "Perfluoroalkyl-selenation of olefins". Tetrahedron Letters. 32 (3): 375–378. doi:10.1016/S0040-4039(00)92632-7.
- ↑ Uneyama, Kenji; Kanai, Masatomi (December 1991). "Generation of perfluoroalkyl radicals at low temperature by tellurolate mediated electron transfer". Tetrahedron Letters. 32 (50): 7425–7426. doi:10.1016/0040-4039(91)80124-O.
- ↑ Rasmusson, Gary H.; Brown, Ronald D.; Arth, Glen E. (March 1975). "Photocatalyzed reaction of trifluoromethyl iodide with steroidal dienones". The Journal of Organic Chemistry. 40 (6): 672–675. doi:10.1021/jo00894a002.
- ↑ Lan-Hargest, Hsuan-Yin; Elliott, John D.; Eggleston, Drake S.; Metcalf, Brian W. (January 1987). "The photochemical rearrangement of a steroidal dienol triflate". Tetrahedron Letters. 28 (52): 6557–6560. doi:10.1016/S0040-4039(00)96912-0.
- 1 2 Miura, Katsukiyo; Takeyama, Yoshihiro; Oshima, Koichiro; Utimoto, Kiitiro (1991). "Triethylborane Induced Perfluoroalkylation of Silyl Enol Ethers and Ketene Silyl Acetals with Perfluoroalkyl Iodides.". Bulletin of the Chemical Society of Japan. 64 (5): 1542–1553. doi:10.1246/bcsj.64.1542.
- ↑ Miura, Katsukiyo; Taniguchi, Masahiko; Nozaki, Kyoko; Oshima, Koichiro; Utimoto, Kiitiro (January 1990). "Triethylborane induced perfluoroalkylation of silyl enol ethers or germyl enol ethers with perfluoroalkyl iodides". Tetrahedron Letters. 31 (44): 6391–6394. doi:10.1016/S0040-4039(00)97073-4.
- ↑ Studer, Armido (3 September 2012). "A "Renaissance" in Radical Trifluoromethylation". Angewandte Chemie International Edition. 51 (36): 8950–8958. doi:10.1002/anie.201202624.
- ↑ Haszeldine, R. N. (1949). "603. The reactions of fluorocarbon radicals. Part I. The reaction of iodotrifluoromethane with ethylene and tetrafluoroethylene". Journal of the Chemical Society (Resumed): 2856. doi:10.1039/JR9490002856.
- 1 2 Langlois, Bernard R.; Billard, Thierry; Roussel, Solveig (February 2005). "Nucleophilic trifluoromethylation". Journal of Fluorine Chemistry. 126 (2): 173–179. doi:10.1016/j.jfluchem.2004.11.007.
- ↑ Prof. Dr. G. K. Surya Prakash, Dr. Fang Wang, Zhe Zhang, Prof. Dr. Ralf Haiges, Dr. Martin Rahm, Prof. Dr. Karl O. Christe, Dr. Thomas Mathew, Prof. Dr. George A. Olah (2014). "Long-Lived Trifluoromethanide Anion: A Key Intermediate in Nucleophilic Trifluoromethylations". Angew. Chem. Int. Ed. 53 (43): 11575 –11578. doi:10.1002/anie.201406505. PMID 25146595.
- ↑ Shono, Tatsuya; Ishifune, Manabu; Okada, Toshio; Kashimura, Shigenori (January 1991). "Electroorganic chemistry. 130. A novel trifluoromethylation of aldehydes and ketones promoted by an electrogenerated base". The Journal of Organic Chemistry. 56 (1): 2–4. doi:10.1021/jo00001a002.
- ↑ Barhdadi, Rachid; Troupel, Michel; Périchon, Jacques (1998). "Coupling of fluoroform with aldehydes using an electrogenerated base". Chemical Communications (12): 1251–1252. doi:10.1039/A801406J.
- ↑ Large, Sylvie; Roques, Nicolas; Langlois, Bernard R. (December 2000). "Nucleophilic Trifluoromethylation of Carbonyl Compounds and Disulfides with Trifluoromethane and Silicon-Containing Bases". The Journal of Organic Chemistry. 65 (26): 8848–8856. doi:10.1021/jo000150s.
- ↑ Fei, Xiang-Shu; Tian, Wei-Sheng; Ding, Kai; Wang, Yun; Qing-Yun, Chen (2010). "NEW, CONVENIENT ROUTE FOR TRIFLUOROMETHYLATION OF STEROIDAL MOLECULES". Org. Synth. 87 (87): 126. doi:10.15227/orgsyn.087.0126.
- ↑ Shibata, N.; Matsnev, A.; Cahard, D. (2010). "Shelf-stable electrophilic trifluoromethylating reagents: A brief historical perspective". Beilstein Journal of Organic Chemistry. 6. doi:10.3762/bjoc.6.65.
- ↑ Umemoto, T. (1996). "Electrophilic Perfluoroalkylating Agents". Chemical Reviews. 96 (5): 1757–1778. doi:10.1021/cr941149u. PMID 11848810.
- 1 2 Barata-Vallejo, S. N.; Lantaño, B.; Postigo, A. (2014). "Recent Advances in Trifluoromethylation Reactions with Electrophilic Trifluoromethylating Reagents". Chemistry: A European Journal. 20 (51): 16806–16829. doi:10.1002/chem.201404005.
- ↑ Yagupolskii, L. M.; Kondratenko, N. V.; Timofeeva, G. N. J. Org. Chem. USSR 1984, 20, 103–106.
- ↑ Teruo, U.; Sumi, I. (1990). "Power-variable trifluoromethylating agents, (trifluoromethyl)dibenzothio- and -selenophenium salt system". Tetrahedron Letters. 31 (25): 3579–3582. doi:10.1016/S0040-4039(00)94447-2.
- ↑ Umemoto, T.; Ishihara, S. (1993). "Power-variable electrophilic trifluoromethylating agents. S-, Se-, and Te-(trifluoromethyl)dibenzothio-, -seleno-, and -tellurophenium salt system". Journal of the American Chemical Society. 115 (6): 2156–2164. doi:10.1021/ja00059a009.
- ↑ Eisenberger, P.; Gischig, S.; Togni, A. (2006). "Novel 10-I-3 Hypervalent Iodine-Based Compounds for Electrophilic Trifluoromethylation". Chemistry: A European Journal. 12 (9): 2579–2586. doi:10.1002/chem.200501052.
- ↑ Kieltsch, I.; Eisenberger, P.; Togni, A. (2007). "Mild Electrophilic Trifluoromethylation of Carbon- and Sulfur-Centered Nucleophiles by a Hypervalent Iodine(III)–CF3 Reagent". Angewandte Chemie International Edition. 46 (5): 754–757. doi:10.1002/anie.200603497.
- ↑ Eisenberger, P.; Kieltsch, I.; Armanino, N.; Togni, A. (2008). "Mild electrophilic trifluoromethylation of secondary and primary aryl- and alkylphosphines using hypervalent iodine(iii)–CF3 reagents". Chemical Communications (13): 1575. doi:10.1039/B801424H.
- ↑ Stanek, K.; Koller, R.; Togni, A. (2008). "Reactivity of a 10-I-3 Hypervalent Iodine Trifluoromethylation Reagent with Phenols". The Journal of Organic Chemistry. 73 (19): 7678–7685. doi:10.1021/jo8014825.
- 1 2 "Preparation of a Trifluoromethyl Transfer Agent: 1-Trifluoromethyl-1,3-Dihydro-3,3-Dimethyl-1,2-Benziodoxole". Organic Syntheses. 88: 168. 2011. doi:10.15227/orgsyn.088.0168.
- ↑ Parsons, A. T.; Buchwald, S. L. (2011). "Copper-Catalyzed Trifluoromethylation of Unactivated Olefins". Angewandte Chemie International Edition. 50 (39): 9120–9123. doi:10.1002/anie.201104053.
- ↑ He, Z.; Luo, T.; Hu, M.; Cao, Y.; Hu, J. (2012). "Copper-Catalyzed Di- and Trifluoromethylation of α,β-Unsaturated Carboxylic Acids: A Protocol for Vinylic Fluoroalkylations". Angewandte Chemie International Edition. 51 (16): 3944–3947. doi:10.1002/anie.201200140.
- ↑ Ma, Jun-An; Cahard, Dominique (December 2004). "Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions". Chemical Reviews. 104 (12): 6119–6146. doi:10.1021/cr030143e.
- ↑ Lin, Jin-Hong; Xiao, Ji-Chang (November 2014). "Recent advances in asymmetric fluorination and fluoroalkylation reactions via organocatalysis". Tetrahedron Letters. 55 (45): 6147–6155. doi:10.1016/j.tetlet.2014.09.031.
- ↑ Skiles, Jerry W.; Fuchs, Victor; Miao, Clara; Sorcek, Ronald; Grozinger, Karl G.; Mauldin, Scott C.; Vitous, Jana; Mui, Philip W.; Jacober, Stephen (February 1992). "Inhibition of human leukocyte elastase (HLE) by N-substituted peptidyl trifluoromethyl ketones". Journal of Medicinal Chemistry. 35 (4): 641–662. doi:10.1021/jm00082a005.
- ↑ Bansal, Romesh C.; Dean, Barbara; Hakomori, Sen-itiroh; Toyokuni, Tatsushi (1991). "Synthesis of trifluoromethyl analogue of L-fucose and 6-deoxy-D-altrose". Journal of the Chemical Society, Chemical Communications (12): 796. doi:10.1039/C39910000796.
- 1 2 Iseki, Katsuhiko; Nagai, Takabumi; Kobayashi, Yoshiro (May 1994). "Asymmetric trifluoromethylation of aldehydes and ketones with trifluoromethyltrimethylsilane catalyzed by chiral quaternary ammonium fluorides". Tetrahedron Letters. 35 (19): 3137–3138. doi:10.1016/S0040-4039(00)76850-X.
- 1 2 Caron, Stéphane; Do, Nga; Arpin, Patrice; Larivée, Alexandre (August 2003). "Enantioselective Addition ofa Trifluoromethyl Anion to Aryl Ketones and Aldehydes". Synthesis. 2003 (11): 1693–1698. doi:10.1055/s-2003-40889.
- ↑ Umemoto, Teruo; Adachi, Kenji (September 1994). "New Method for Trifluoromethylation of Enolate Anions and Applications to Regio-, Diastereo- and Enantioselective Trifluoromethylation". The Journal of Organic Chemistry. 59 (19): 5692–5699. doi:10.1021/jo00098a030.
- ↑ Nagib, David A.; Scott, Mark E.; MacMillan, David W. C. (2009). "Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis". Journal of the American Chemical Society. 131 (31): 10875–10877. doi:10.1021/ja9053338. PMC 3310169
 . PMID 19722670.
. PMID 19722670. - ↑ Allen, Anna E.; MacMillan, David W. C. (2010). "The Productive Merger of Iodonium Salts and Organocatalysis: A Non-photolytic Approach to the Enantioselective α-Trifluoromethylation of Aldehydes". Journal of the American Chemical Society. 132 (14): 4986–4987. doi:10.1021/ja100748y. PMID 20297822.
- ↑ Deng, Qing-Hai; Wadepohl, Hubert; Gade, Lutz H. (2012). "Highly Enantioselective Copper-Catalyzed Electrophilic Trifluoromethylation of β-Ketoesters". Journal of the American Chemical Society. 134 (26): 10769–10772. doi:10.1021/ja3039773. PMID 22693950.
- ↑ Herrmann, Aaron T.; Smith, Lindsay L.; Zakarian, Armen (2012). "A Simple Method for Asymmetric Trifluoromethylation of-Acyl Oxazolidinones via Ru-Catalyzed Radical Addition to Zirconium Enolates". Journal of the American Chemical Society. 134 (16): 6976–6979. doi:10.1021/ja302552e. PMID 22486383.