Systole
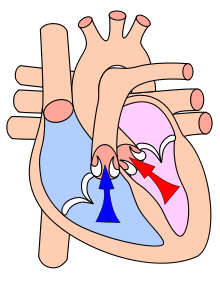

Systole /ˈsɪstəliː/ is the part of the cardiac cycle when the ventricles contract.[1] The term "systole" originates from New Latin, from Ancient Greek συστολή (sustolē), from συστέλλειν (sustellein, "to contract"), from σύν (syn, "together") + στέλλειν (stellein, "send").
The mammalian heart has 4 chambers: the left atrium, the left ventricle, the right atrium and the right ventricle.
When the smaller, upper atria chambers contract in late diastole, they send blood down to the larger, lower ventricle chambers. When the lower chambers are filled and the valves to the atria are closed, the ventricles undergo isovolumetric contraction (contraction of the ventricles while all valves are closed), marking the first stage of systole. The second phase of systole sends blood from the left ventricle to the aorta and body extremities, and from the right ventricle to the lungs. Thus, the atria and ventricles contract in alternating sequence. The left and right atria feed blood, at the same time, into the ventricles. Then, the left and right ventricles contract simultaneously as well.
Cardiac systole is the contraction of the cardiac muscle in response to an electrochemical stimulus to the heart's cells (cardiomyocytes).
The cardiac output (CO) is the volume of blood pumped by the left ventricle in one minute. The ejection fraction (EF) is the volume of blood pumped divided by the total volume of blood in the left ventricle.[2]
Types
Electrical systole
Cardiac electrical systole is staged and first derived from sympathetic charge from the sinoatrial node (SA node). Subsequent physiologic discharge from the SA node then finds its way through the atrial mass, eventually meeting at the atrioventricular node to be gated through the available channels from the atria to the ventricles to allow ventricular systole or [LVEF] + [RVEF].
Mechanical systole
Electrical systole opens voltage-gated sodium, potassium and calcium channels. A rise in intracellular calcium triggers the interaction of actin and myosin in the presence of ATP that generates force (see Physiological mechanism below). The muscular contraction of myocardium generates an active stress that increases intra-ventricular pressure and when intra-ventricular pressure exceeds aortic pressure there is ejection of blood. Mechanical systole is the origin of the pulse. The pulse is readily palpated (felt) at many points on the body and represents a universally accepted tactile (and sometimes visual) method of observing peak or systolic blood pressure. Mechanical forces resulting from electrical systole cause rotation of the muscle mass around the long and short axes; a process envisaged as "wringing" of the ventricles.
Atrial systole
Atrial systole represents the contraction of myocardium of the left and right atria. Atrial systole occurs late in ventricular diastole. One force driving blood from the atria to the ventricles is the decrease in ventricular pressure that occurs during ventricular diastole. The drop in ventricular pressure that occurs during ventricular diastole allows the atrioventricular valves to open, emptying the contents of the atria into the ventricles. Contraction of the atrium confers a relatively minor, additive effect toward ventricular filling; atrial contraction becomes significant in left ventricular hypertrophy, in which the ventricle does not fully relax during ventricular diastole. Loss of normal electrical conduction in the heart, as seen during atrial fibrillation, atrial flutter, and complete heart block, may abolish atrial systole. The aortic valve and pulmonary valve remain closed, while the atrioventricular mitral and tricuspid valves remain open because the pressure gradient between the atrium and ventricle is preserved during late ventricular diastole.
Contraction of the atria follows depolarization, represented by the P wave of the ECG. As the atrial muscles contract from the superior portion of the atria toward the atrioventricular septum, pressure rises within the atria and blood is pumped into the ventricles through the open atrioventricular (tricuspid, and mitral or bicuspid) valves. At the start of atrial systole, the ventricles are normally filled with approximately 70–80 percent of their capacity due to inflow during diastole. Atrial contraction, also referred to as the "atrial kick," contributes the remaining 20–30 percent of filling. Atrial systole lasts approximately 100 ms and ends prior to ventricular systole, as the atrial muscle returns to diastole.[3]
The ventricles are histologically and electrically isolated from the atria by the unique and electrically impermeable collagen layers of connective tissue known as the cardiac skeleton. The bulwarks of this entity stem from the central body to form the four valve rings. Collagen extensions from the valve rings seal and limit atrial electrical influence from ventricular electrical influence to the SA/AV/Purkinje pathways. Exceptions such as accessory pathways may occur in this firewall between atrial and ventricular electrical influence but are rare. The compromised load of atrial fibrillation detracts from overall performance but the ventricles continue to work as a physiologically effective pump. Given this pathology, ejection fraction may deteriorate by ten to thirty percent. Uncorrected atrial fibrillation can lead to heart rates approaching 200 beats per minute. If one can slow this rate down to a normal range of approximately 80 beats per minute, the filling time of the heart cycle is longer and confers additional benefit to the pumping ability of the heart. Breathless individuals with uncontrolled atrial fibrillation can be rapidly returned to normal breathing when conversion with medication or electrical cardioversion is attempted. Pharmacological manipulation of rate control, for example, by beta blocker|beta adrenoceptor antagonists, non-dyhydropyridine calcium channel blockers and digoxin are important historical interventions in this condition. Individuals prone to a hypercoagulable state are at a decided risk of thromboembolism, thus requiring therapy with warfarin for life if the defined pathology cannot be corrected.
Right atrial systole
Right atrial systole coincides with right ventricular diastole, driving the blood through the tricuspid valve (TV), into the right ventricle. The time variable of right atrial systole, is (TV) open to (TV) close.
Left atrial systole
Left atrial systole coincides with left ventricular diastole, driving blood through the mitral valve (MV) (also known as the bicuspid valve), into the left ventricle. The time variable of left atrial systole is (MV) open to (MV) close. The atria contains two valves, the mitral (bicuspid) and the tricuspid valves which open during the late stages of diastole.
Atrial fibrillation
Atrial fibrillation represents a common electrical malady apparent during the time interval of atrial systole. Theory suggests that an ectopic focus, usually within the pulmonary trunks, competes with the sinoatrial node for electrical control of the atrial chambers to the detriment of atrial myocardial performance. Ordered sinoatrial control of atrial electrical activity is lost, as a result coordinated pressure generation does not occur in the upper cardiac chambers. Atrial fibrillation represents an electrically disordered but well blood perfused atrial mass working in an uncoordinated fashion with an electrically (comparatively) healthy ventricle.
Ventricular systole

Ventricular systole is a written description of the contraction of the myocardium of the left and right ventricles. Ventricular systole induces increased pressure in the left and right ventricles. Pressure in the ventricles rises to a level above that of the atria, thus closing the tricuspid and mitral valves, which are prevented from inverting by chordae tendineae and associated papillary muscles. Ventricular pressure continues to rise in isovolumetric contraction with maximal pressure generation (max dP/dt) occurring during this phase, until the pulmonary and aortic valves open in the ejection phase. In the ejection phase, blood flows down its pressure gradient through the aorta and pulmonary artery from left and right ventricles respectively. It is important to note that cardiac muscle perfusion through coronary vessels does not occur during ventricular systole, but occurs during ventricular diastole.
Ventricular systole is the origin of the pulse.
Right ventricular systole
Right ventricular systole drives blood through the pulmonary valve (PV) into the lungs. Right ventricular systole is volumetrically defined as right ventricular ejection fraction (RVEF). The time variable of right ventricular systole is PV open to PV close. Increased RVEF is indicative of pulmonary hypertension.
Left ventricular Systole
Left ventricular systole drives blood through the aortic valve (AoV) to the body and organs excluding the lungs. Left ventricular systole is volumetrically defined as left ventricular ejection fraction (LVEF). The time variable of left ventricular systole is AoV open to AoV close.
Physiological mechanism
Systole of the heart is initiated by the electrically excitable cells of the sinoatrial node. These cells are activated spontaneously by depolarization of their representative membranes beyond a given threshold for excitation. At this point, voltage-gated calcium channels on the cell membrane open and allow calcium ions to pass through, into the sarcoplasm of the cardiac muscle cell. Calcium ions bind to ryanodine receptors on the sarcoplasmic reticulum causing a flux of calcium ions to the sarcoplasm.
Calcium ions bind to troponin C, causing a conformational change in the troponin-tropomyosin complex, and thus allowing myosin head binding sites on F-Actin to be exposed. This transition allows cross bridge cycling to occur. The cardiac action potential spreads distally to the small branches of the Purkinje tree via the flux of cations through gap junctions that connect the sarcoplasm of adjacent myocytes. The electrical activity of ventricular systole is coordinated by the atrioventricular node, this discrete collection of cells receives electrical stimulation from the atrium, but also has a slower intrinsic pacemaker activity. The cardiac action potential is propagated down the bundle of His to Purkinje fibres which rapidly causes coordinated depolarisation, and excitation-contraction coupling from the apex of the heart up to the roots of the great vessels.
Clinical notation
When blood pressure is stated for medical purposes, it is usually written with the systolic and diastolic pressure separated by a slash; for example: 120/80 mmHg. This has nothing to do with the mathematical notation for a fraction or ratio: it is not a display of a numerator over a denominator but rather a medical notation used for quickly showing the two clinically significant pressures involved and cannot be reduced into lower terms.
See also
References
- ↑ Simmers, Louise (2004). Introduction to Health Science Technology. Australia: Thomson/Delmar Learning. p. 169. ISBN 9781401811280.
- ↑ Lang RM, Bierig M, Devereux RB, et al. (March 2006). "Recommendations for chamber quantification". Eur J Echocardiogr. 7 (2): 79–108. doi:10.1016/j.euje.2005.12.014. PMID 16458610.
- ↑ Betts, J. Gordon (2013). Anatomy & physiology. pp. 787–846. ISBN 1938168135. Retrieved 11 August 2014.