Soil-transmitted helminthiasis

Soil-transmitted helminthiasis (STH) is a type of helminth infection (helminthiasis) caused by different species of roundworms. It is caused specifically by those worms which are transmitted through soil contaminated with faecal matter and are therefore called soil-transmitted helminths. Three types of soil-transmitted helminthiasis can be distinguished: ascariasis, hookworm disease and trichuriasis. These three types of infection are therefore caused by the large roundworm A. lumbricoides; the hookworms Necator americanus or Ancyclostoma duodenale; and by the whipworm Trichuris trichiura respectively.
It has become the most common parasitic disease of humans worldwide. Approximately two billion people (about a third of global population) are infected as of the latest estimate, and four billion at risk, surpassing even the all-time most prevalent parasitic disease, malaria.[1] The largest numbers of cases occur in impoverished rural areas of Subsaharan Africa, Latin America, Southeast Asia, and China.[2] Its main cause, like for many types of helminth infections, is lack of sanitation, such as the practice of open defecation and lack of hygiene such as hand washing.[3][4] It is regarded as one of the world's most important causes of intellectual and physical retardation.[5]
The helminthic disease is so named because the infection is transmitted through ingestion of the nematode eggs in the soil, which is contaminated through excrements. Therefore, the disease is most prevalent in warm and moist climates where sanitation and hygiene are poor and waters are unsafe, including the temperate zones during warmer months. STH is categorised among Neglected Tropical Diseases because it inflicts tremendous disability and suffering, which can be clinically treated and relatively easily be prevented (primarily through improved sanitation), yet negligible attention has been given for many years.[6] It is now among the target diseases of London Declaration on Neglected Tropical Diseases (launched on 30 January 2012) to be controlled/eradicated by 2020.[7]
Simple prevention and control strategies are access to improved sanitation, public awareness on personal hygiene and health education.
Signs and symptoms
Symptoms becomes evident only when the intensity of infection is relatively high. Thus the degree of negative outcomes is directly related to worm burden; more worms means greater severity of disease.
General
Most conditions of STH have a light worm burden and usually have no discernible symptoms. Heavy infections however cause a range of health problems, including abdominal pain, diarrhoea, blood and protein loss, rectal prolapse, and physical and mental retardation.
Severe ascariasis is typically a pneumonia, as the larvae invades lungs, producing fever, cough and dyspnoea during early stage of infection.
Hookworm infections insinuate a skin reaction (dermatitis), increased white blood cells (eosinophils), a pulmonary reaction (pneumonitis), and skin rash (urticarial).
Iron deficiency anaemia due to blood loss is a common symptom.[8]
Malnutrition
STH is often associated with malnutrition in children as it worsens the nutritional status of the people they infect in multiple ways.[1] The worms can induce intestinal bleeding, competition for nutrients (malabsorption of nutrients), frequent anaemia and diarrhoea.[9] Soil-transmitted helminths can also cause loss of appetite.[1] These nutritional "knock on" effects of STH can have a significant impact on growth and physical development of children. In endemic countries, communities remain suppressed due to malnourishment, cognitive disability and physical weaknesses as a result of heavy infections.
Types
STHs are essentially intestinal parasites and their eggs are liberated along the faeces of infected persons into the soil. Ascaris and hookworm eggs become infective as they develop into larvae in soil. Infection occurs when vegetables and fruits, contaminated with soil infested eggs, are consumed; or when hands or fingers have been contaminated with dirt carrying the eggs are put in the mouth. On the other hand, hookworm eggs are not directly infective. They hatch in soil, releasing mobile larvae that can penetrate the skin. Thus infection is acquired through accidental contact with contaminated soil.[5]
Ascariasis
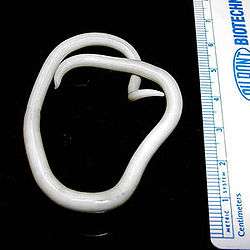
Ascariasis of STH is caused by the large roundworm A. lumbricoides. It is estimated to be the most widespread STH, affecting approximately 1 billion people. The victims constitute about half of the populations in tropical and subtropical areas. Most conditions are mild and often show little or no symptoms. Heavy infections however are debilitating, causing severe intestinal blockage and impair growth in children. Children, compounded with malnutrition, are most infected, with the most common age group being 3 to 8 year olds, with an annual death of about 20,000. Children are more susceptible due to their frequent exposure to contaminated environment such as during playing, eating raw vegetables and fruits, and drinking wastewater.[5]
Hookworm disease

Hookworm infection of STH is caused by N. americanus and A. duodenale. Mild infections produce diarrhoea and abdominal pain. More severe infections can create serious health problems for newborns, children, pregnant women, and malnourished adults. In fact it is the leading cause of anaemia and protein deficiency in developing nations, afflicting an estimated 740 million people. N. americanus is the more common hookworm, while A. duodenale is more geographically restricted. Unlike other STHs, in which school-age children are most affected, high-intensity hookworm infections are more frequent in adults, specifically women. Roughly, 44 million pregnant women are estimated to be infected. The disease causes severe adverse effects in both the mother and infant, such as low birth weight, impaired milk production, and increased risk of mortality.[5]
Trichuriasis

Whipworm (Trichuris trichiura) is the third most common STH-causing nematode in humans. According to current estimate, nearly 800 million people are infected, and majority of the victims are children. Heavy infections could lead to acute symptoms such as diarrhoea and anaemia, and chronic symptoms such as growth retardation and impaired cognitive development. Medical conditions are more often serious since coinfection with protozoan parasites such as Giardia and Entamoeba histolytica, and with other nematodes is common.[5] Predominantly a tropical disease of developing countries, trichuriasis is quite common in the United States.[10]
Diagnosis
For basic diagnosis, specific helminths can be generally identified from the faeces, and their eggs microscopically examined and enumerated using fecal egg count method. However, there are certain limitations such as the inability to identify mixed infections, and on clinical practice, the technique is inaccurate and unreliable.[11][12] A novel effective method for egg analysis is the Kato-Katz technique. It is a highly accurate and rapid method for A. lumbricoides and T. trichiura; however not so much for hookworm, which could be due to fast degeneration of the rather delicate hookworm eggs.[13]
Prevention
Prevention and control measures to prevent soil-transmitted helminthiasis are the following: availability of clean water for personal and domestic uses, improved access to sanitation which includes the use of properly functioning and clean toilets by all community members, education on personal hygiene such as hand washing and hygienic and safe food preparation; eliminating the use of untreated human faeces as fertilizer.[1]
Treatment
The World Health Organizations recommended albendazole or mebendazole for treatment.[1]
Mass treatment with drugs
One strategy to control the disease in areas where it is common is the treatment of entire groups of people regardless of symptoms via mass drug administration. This is often done among school-age children and is known as deworming.[1][14] While testing and treating children who are infected looks like it is effective, there is insufficient evidence to conclude that routine deworming, in the absence of a positive test, improves nutrition, haemoglobin, school attendance or school performance.[15]
For this purpose, broad-spectrum benzimidazoles such as mebendazole and albendazole are the drugs of choice recommended by WHO. These anthelminthics are administered in a single dose are safe, relatively inexpensive, and effective for several months. Mebendazole can be given with a single dose twice a day for three consecutive days. Albendazole is given at a single dose. WHO recommends annual treatment in areas where between 20 and 50% of people are infected, and a twice a year treatment if it is over 50%; and in low risk situation (i.e. less than 20% prevalence) case-by-case treatment.[8][16] In addition to these, pyrantel pamoate is also equally effective on ascaris. However, it has been reported that albendazole, mebendazole, and pyrantel pamoate are not entirely effective against T. trichiura with single oral doses in population-based control.[17]
Drugs for those with other diseases
In cases of coinfection, combination therapy with ivermectin and diethylcarbamazine is advocated. Indeed, with coinfection with malaria and HIV, especially among African women, the current regimes for controlling STHs are inadequate.[18] It is more pressing for trichuriasis that the recommended drugs fail to provide positive results.[19] A novel drug tribendimidine, which was approved by Chinese authorities for human use in 2004, has been subjected to clinical trials showing that they are highly effective against major human flukes, ascaris (>90% cure rate) and hookworm (>82%); however with low cure rate for whipworm (<37%).[20]
Surgical intervention
In some cases with severe infestation the worms, such as Ascaris, may cause bowel obstruction, requiring emergency surgery.[21] The bowel obstruction may be due to all the worms or twisting of the bowel.[21] During the surgery the worms may be manually removed.[21]
Epidemiology
Regions
Infections are widely distributed in tropical and subtropical areas, with the greatest numbers occurring in sub-Saharan Africa, the Americas, China and east Asia.[1]
Infection estimates
The World Health Organisation estimates that globally more than 1.5 billion people (24% of the total population) have a soil-transmitted helminth infection.[1] Over 270 million preschool-age children and over 600 million school-age children live in areas where these parasites are intensively transmitted, and are in need of treatment and preventive interventions. Latest estimates indicate that more than 880 million children are in need of treatment from STH infections.[2][9][22]
By type of parasitic worm the breakdown is:[23]
- approximately 807-1,121 million with ascaris
- approximately 576-740 million with hookworm
- approximately 604-795 million with whipworm
Deaths
Latest estimates indicate that the total annual death toll which is directly attributable is as high as 135,000.[2][9][22] The death toll due to the malnutrition link is likely to be much higher.
References
- 1 2 3 4 5 6 7 8 "Soil-transmitted helminth infections Fact sheet N°366". who.int. April 2014. Retrieved 18 October 2014.
- 1 2 3 WHO. Eliminating Soil-transmitted Helminthiasis as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020 (PDF). WHO Press, World Health Organization, Geneva, Switzerland. pp. 1–78. ISBN 978-92-4-150312-9.
- ↑ Ziegelbauer, Kathrin (2012). "Effect of sanitation on soil-transmitted helminth infection: Systematic review and meta-analysis". PLOS Medicine. 9: e1001162. doi:10.1371/journal.pmed.1001162. PMC 3265535
 . PMID 22291577.
. PMID 22291577. - ↑ Strunz, Eric C. (2014). "Water, Sanitation, Hygiene, and Soil-Transmitted Helminth Infection: A Systematic Review and Meta-Analysis". PLOS Medicine. 11: e1001620. doi:10.1371/journal.pmed.1001620.
- 1 2 3 4 5 Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ (2006). "Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm". The Lancet. 367 (9521): 1521–1532. doi:10.1016/S0140-6736(06)68653-4. PMID 16679166.
- ↑ "Neglected Tropical Diseases". cdc.gov. June 6, 2011. Retrieved 28 November 2014.
- ↑ London Declaration (2012) (30 January 2012). "London Declaration on Neglected Tropical Diseases" (PDF). Retrieved 2013-03-26.
- 1 2 WHO (2012). Helminth Control in School-age Children: a Guide for Managers of Control Programmes (PDF) (2 ed.). WHO Press, World Health Organization, Geneva, Switzerland. pp. 1–75. ISBN 978-92-4-154826-7.
- 1 2 3 Yap P, Fürst T, Müller I, Kriemler S, Utzinger J, Steinmann P (2012). "Determining soil-transmitted helminth infection status and physical fitness of school-aged children". Journal of Visualized Experiments. 66: e3966. doi:10.3791/3966. PMID 22951972.
- ↑ Starr MC, Montgomery SP (2011). "Soil-transmitted helminthiasis in the United States: a systematic review--1940-2010". Am J Trop Med Hyg. 85 (4): 680–684. doi:10.4269/ajtmh.2011.11-0214. PMC 3183777
 . PMID 21976572.
. PMID 21976572. - ↑ Humphries D, Nguyen S, Boakye D, Wilson M, Cappello M (2012). "The promise and pitfalls of mass drug administration to control intestinal helminth infections". Curr Opin Infect Dis. 25 (5): 584–589. doi:10.1097/QCO.0b013e328357e4cf. PMID 22903231.
- ↑ Krauth SJ, Coulibaly JT, Knopp S, Traoré M, N'Goran EK, Utzinger J (2012). "An in-depth analysis of a piece of shit: distribution of Schistosoma mansoni and hookworm eggs in human stool". PLoS Negl Trop Dis. 6 (12): e1969. doi:10.1371/journal.pntd.0001969. PMC 3527364
 . PMID 23285307.
. PMID 23285307. - ↑ Tarafder MR, Carabin H, Joseph L, Balolong E Jr, Olveda R, McGarvey ST (2010). "Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a 'gold standard'". PLoS Negl Trop Dis. 40 (4): 399–404. doi:10.1016/j.ijpara.2009.09.003. PMC 2829363
 . PMID 19772859.
. PMID 19772859. - ↑ Mascarini-Serra L (2011). "Prevention of soil-transmitted helminth infection". Journal of Global Infectious Diseases. 3 (2): 175–182. doi:10.4103/0974-777X.81696. PMC 3125032
 . PMID 21731306.
. PMID 21731306. - ↑ Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P (2012). "Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance". Cochrane Database Syst Rev. 7 (7): CD000371. doi:10.1002/14651858.CD000371.pub4. PMID 22786473.
- ↑ WHO (2006). Preventive Chemotherapy in Human Helminthiasis : Coordinated Use of Anthelminthic Drugs in Control Interventions : a Manual for Health Professionals and Programme Managers (PDF). WHO Press, World Health Organization, Geneva, Switzerland. pp. 1–61. ISBN 9241547103.
- ↑ Keiser J, Utzinger J (2008). "Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis". JAMA. 299 (16): 1937–1948. doi:10.1001/jama.299.16.1937. PMID 18430913.
- ↑ Ivan E, Crowther NJ, Rucogoza AT, Osuwat LO, Munyazesa E, Mutimura E, Njunwa KJ, Zambezi KJ, Grobusch MP (2012). "Malaria and helminthic co-infection among HIV-positive pregnant women: prevalence and effects of antiretroviral therapy". Acta Tropica. 124 (3): 179–184. doi:10.1016/j.actatropica.2012.08.004. PMID 22940013.
- ↑ Speich B, Ame SM, Ali SM, Alles R, Hattendorf J, Utzinger J, Albonico M, Keiser (2012). "Efficacy and safety of nitazoxanide, albendazole, and nitazoxanide-albendazole against Trichuris trichiura infection: a randomized controlled trial". PLoS Negl Trop Dis. 6 (6): e1685. doi:10.1371/journal.pntd.0001685. PMC 3367984
 . PMID 22679525.
. PMID 22679525. - ↑ Xiao SH, Utzinger J, Tanner M, Keiser J, Xue J (2013). "Advances with the Chinese anthelminthic drug tribendimidine in clinical trials and laboratory investigations". Acta Tropica. 126 (2): 115–126. doi:10.1016/j.actatropica.2013.01.009. PMID 23352956.
- 1 2 3 Hefny, AF; Saadeldin, YA; Abu-Zidan, FM (May 2009). "Management algorithm for intestinal obstruction due to ascariasis: a case report and review of the literature.". Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery : TJTES. 15 (3): 301–5. PMID 19562557.
- 1 2 Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG (2012). "A research agenda for helminth diseases of humans: the problem of helminthiases". PLoS Negl Trop Dis. 6 (4): e1582. doi:10.1371/journal.pntd.0001582. PMC 3335854
 . PMID 22545164.
. PMID 22545164. - ↑ Hotez PJ, Fenwick A, Savioli L, Molyneux DH (2009). "Rescuing the bottom billion through control of neglected tropical diseases". The Lancet. 373 (9674): 1570–1575. doi:10.1016/S0140-6736(09)60233-6. PMID 19410718.