Radiocontrast agent
Radiocontrast agents are a type of medical contrast medium used to improve the visibility of internal bodily structures in X-ray-based imaging techniques such as computed tomography (CT), radiography, and fluoroscopy. Radiocontrast agents are typically iodine or barium compounds. When an agent improves visibility of an area, it is called "contrast enhancing".
Magnetic resonance imaging (MRI) functions through different principles and thus utilizes different contrast agents. These compounds work by altering the magnetic properties of nearby hydrogen nuclei.
Types and uses
Radiocontrast agents used in X-ray examinations can be grouped based on its use.
Iodinated (intravascular)

Iodine-based contrast media are usually classified as ionic or non-ionic. Both types are used most commonly in radiology due to their relatively harmless interaction with the body and its solubility. Contrast media are primarily used to visualize vessels and changes in tissues on radiography and CT. Contrast media can also be used for tests of the urinary tract, uterus and fallopian tubes. It may cause the patient to feel as if he or she has urinated on him- or herself. It also puts a metallic taste in the mouth of the patient.
Modern intravenous contrast agents are typically based on iodine. This may be bound either in an organic (non-ionic) compound or an ionic compound. Ionic agents were developed first and are still in widespread use depending on the requirements, but may result in additional complications. Organic agents which covalently bind the iodine have fewer side effects as they do not dissociate into component molecules. Many of the side effects are due to the hyperosmolar solution being injected. i.e. they deliver more iodine atoms per molecule. The more iodine, the more "dense" the X-ray effect.
Organic iodine molecules used for contrast include iohexol, iodixanol and ioversol. Iodine-based contrast media are water-soluble. These contrast agents are sold as clear, colorless water solutions, with the concentration usually expressed as mg I/ml. Modern iodinated contrast agents can be used almost anywhere in the body. Most often they are used intravenously, but for various purposes they can also be used intraarterially, intrathecally (as in diskography of the spine) and intraabdominally – just about any body cavity or potential space.
Iodine contrast agents are used for the following:
- Angiography (arterial investigations)
- Venography (venous investigations)
- VCUG (voiding cystourethrography)
- HSG (hysterosalpingogram)
- IVU (intravenous urography)
Ionic
Ionic contrast media typically, but not always, have higher osmolality and more side-effects.
| Compound | Name | Type | Iodine content | Osmolality | |
|---|---|---|---|---|---|
| Ionic | diatrizoate (Hypaque 50) | Monomer | 300 mgI/ml | 1550 | High |
| Ionic | metrizoate (Isopaque 370) | Monomer | 370 mgI/ml | 2100 | High |
| Ionic | iothalamate (Conray) | 600-2400 | High | ||
| Ionic | ioxaglate (Hexabrix) | Dimer | 320 mgI/ml | 580 | Low |
Non-ionic
Non-ionic contrast media have lower osmolality and tend to have fewer side-effects.
| Compound | Name | Type | Iodine content | Osmolality | |
|---|---|---|---|---|---|
| Non-ionic | iopamidol (Isovue 370) | Monomer | 370 mgI/ml | 796 | Low |
| Non-ionic | iohexol (Omnipaque 350) | Monomer | 350 mgI/ml | 884 | Low |
| Non-ionic | ioxilan (Oxilan 350) | Monomer | 350 mgI/ml | 695 | Low |
| Non-ionic | iopromide (Ultravist 370) | Monomer | 370 mgI/ml | 774 | Low |
| Non-ionic | iodixanol (Visipaque 320) | Dimer | 320 mgI/ml | 290 | Low |
| Non-ionic | ioversol | ||||
Barium (gastro-intestinal)
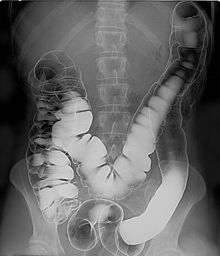
Barium sulfate is mainly used in the imaging of the digestive system. The substance exists as a water-insoluble white powder that is made into a slurry with water and administered directly into the gastrointestinal tract.
- Barium enema (large bowel investigation) and DCBE (double contrast barium enema)
- Barium swallow (oesophageal investigation)
- Barium meal (stomach investigation) and double contrast barium meal
- Barium follow through (stomach and small bowel investigation)
- CT pneumocolon / virtual colonoscopy
Barium sulfate, an insoluble white powder is typically used for enhancing contrast in the GI tract. Depending on how it is to be administered the compound is mixed with water, thickeners, de-clumping agents, and flavourings to make the contrast agent. As the barium sulfate doesn't dissolve, this type of contrast agent is an opaque white mixture. It is only used in the digestive tract; it is usually swallowed or administered as an enema. After the examination, it leaves the body with the feces.
Air
As in the picture on the right where both air and barium are used together (hence the term "double-contrast" barium enema) air can be used as a contrast material because it is less radio-opaque than the tissues it is defining. In the picture it highlights the interior of the colon. An example of a technique using purely air for the contrast medium is an air arthrogram where the injection of air into a joint cavity allows the cartilage covering the ends of the bones to be visualized.
Before the advent of modern neuroimaging techniques, air or other gases were used as contrast agents employed to displace the cerebrospinal fluid in the brain while performing a pneumoencephalography. Sometimes called an "air study", this once common yet highly-unpleasant procedure was used to enhance the outline of structures in the brain, looking for shape distortions caused by the presence of lesions.
Carbon dioxide
Carbon dioxide also has a role in angiography. It is low-risk as it is a natural product with no risk of allergic potential. However, it can be used only below the diaphragm as there is a risk of embolism in neurovascular procedures. It must be used carefully to avoid contamination with room air when injected. It is a negative contrast agent in that it displaces blood when injected intravascularly.
Other
An older type of contrast agent, Thorotrast was based on thorium dioxide. While it provided excellent image enhancement, its use was abandoned since it turned out to be highly carcinogenic, unfortunately though not before having been administered to millions of patients prior to being disused.
Adverse effects
Modern iodinated contrast agents are generally well tolerated.[1] The major side effects of radiocontrast are anaphylactoid reactions and contrast-induced nephropathy.
Anaphylactoid reactions
Anaphylactoid reactions occur rarely,[2][3][4] but can occur in response to injected as well as oral and rectal contrast and even retrograde pyelography. They are similar in presentation to anaphylactic reactions, but are not caused by an IgE-mediated immune response. Patients with a history of contrast reactions, however, are at increased risk of anaphylactoid reactions.[5][6] Pretreatment with corticosteroids has been shown to decrease the incidence of adverse reactions.[7][8]
Anaphylactoid reactions range from urticaria and itching, to bronchospasm and facial and laryngeal edema. For simple cases of urticaria and itching, an oral or intravenous antihistamine such as diphenhydramine is appropriate. For more severe reactions, including bronchospasm and facial or neck edema, albuterol inhaler, or subcutaneous or IV epinephrine, plus diphenhydramine may be needed. If respiration is compromised, an airway must be established prior to medical management.
Anaphylaxis to ionic (high osmolar) contrast agent injections that occurred in two clusters of reactions on two occasions (1983 and 1987) in a single radiology clinic in London Ontario. On each occasion, these anaphylactic reactions were associated with contamination of the injection by natural rubber components (disposable plastic syringes in the first case and rubber ampoule seals in the second case). The allergenic-toxic rubber leachate was MBT (mercaptobenzothiazole). This is a known allergen that becomes bound to plasma proteins, creating a hapten-protein complex – a signature mechanism in true IgE drug allergy and true anaphylactic reactions (not "anaphylactoid" reactions).
A Japanese syringe manufacturer, Terumo, implicated in syringe-related toxic laboratory cell culture effects in Australia in 1981, was instrumental in pro-actively making Japanese disposable syringes and ampoule seals free of natural rubber. Katayama's 1990 article in Radiology showed that a new type of nonionic (low osmolar) contrast agent was associated with significantly fewer severe life-threatening reactions than the older ionic (high osmolar) contrast agents.[9] By merchandizing the Katayama series reprints, manufacturers persuaded users worldwide to switch to the almost exclusive use of the expensive nonionic agents.
What was unknown to the Katayama researchers was that the ampoule seals of the "safer" nonionic contrast agents were made from artificial rubber, whereas the ionic agents were sealed with natural rubber. In 1987, it was the leaching of allergenic MBT from the rubber seals of ionic ampoules that caused a series of allergic reactions (including anaphylaxis) in a radiology office in Canada.[10] The worldwide hazard of MBT contamination of injections was unknown then and, as the World Health Organization reported it remains as an unknown hazard still – after three decades.[11]
The most significant study, proving that injections of ionic (high osmolar) agents are at least as safe as the newer, very expensive nonionics was published in Radiology in 1997.[12] Lasser did not comment that the marked drop in the incidence of severe reactions with ionic agents was related to the removal of natural rubber contamination from ionic ampoule seals.
Contribution of seafood and other allergies
Please omit the term "iodine allergy", because this kind of allergy does not exist.[13] Suspicion of seafood "allergy" is not a sufficient contraindication to the use of iodinated contrast material. While iodine levels in seafood are higher than in non-seafood items, the consumption of the latter exceeds that of the former by far and there is no evidence that the iodine content of seafood is related to reactions to seafood.[14] Available data suggest that seafood allergy increases the risk of a contrast-mediated reaction by approximately the same amount as allergies to fruits or those with asthma.[15] In other words, over 85% of patients with seafood allergies will not have an adverse reaction to iodinated contrast.[14] Finally, there is no evidence that adverse skin reactions to iodine-containing topical antiseptics (e.g., povidone-iodine) are of any specific relevance to administration of I.V. contrast material.[14][16]
Contrast-induced nephropathy
Contrast-induced nephropathy is defined as either a greater than 25% increase of serum creatinine or an absolute increase in serum creatinine of 0.5 mg/dL.[17]
Effects on thyroid function
A 2012 paper in the Journal of the American Medical Association reports that "Iodinated contrast media exposure is associated with subsequent development of incident hyperthyroidism and incident overt hypothyroidism."
Drug interactions
It has been recommended[18] that metformin, an oral antidiabetic agent, be stopped for 48 hours following the intravascular administration of contrast media and that the use of metformin not be resumed until renal function has been shown to be normal. The reasoning is that if the contrast medium causes kidney failure (as happens rarely) and the person continues to take metformin (which is normally excreted by the kidneys), there may be a toxic accumulation of metformin, increasing the risk of lactic acidosis, a dangerous complication.
However, guidelines published by the Royal College of Radiologists suggest this is not as important for patients who receive less than 100 ml of contrast media and have normal renal function. If renal impairment is found before administration of the contrast, metformin should be stopped 48 hours before and after the procedure.[19]
See also
- Contrast medium
- Power injector
References
| Wikimedia Commons has media related to Radiocontrast agents. |
- ↑ Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- ↑ Karnegis JN, Heinz J (1979). "The risk of diagnostic cardiovascular catheterization". Am Heart J. 97 (3): 291–7. doi:10.1016/0002-8703(79)90427-7. PMID 420067.
- ↑ Lasser EC, Berry CC, Talner LB, Santini LC, Lang EK, Gerber FH, Stolberg HO (1987). "Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material". N Engl J Med. 317 (14): 845–9. doi:10.1056/NEJM198710013171401. PMID 3627208.
- ↑ Greenberger PA, Patterson R, Tapio CM (1985). "Prophylaxis against repeated radiocontrast media reactions in 857 cases. Adverse experience with cimetidine and safety of beta-adrenergic antagonists". Arch Intern Med. 145 (12): 2197–200. doi:10.1001/archinte.145.12.2197. PMID 2866755.
- ↑ Greenberger PA, Patterson R (1988). "Adverse reactions to radiocontrast media". Prog Cardiovasc Dis. 31 (3): 239–48. doi:10.1016/0033-0620(88)90017-5. PMID 3055068.
- ↑ Lang DM, Alpern MB, Visintainer PF, Smith ST (1993). "Elevated risk of anaphylactoid reaction from radiographic contrast media is associated with both beta-blocker exposure and cardiovascular disorders". Arch Intern Med. 153 (17): 2033–40. doi:10.1001/archinte.153.17.2033. PMID 8102844.
- ↑ Lasser EC, Berry CC, Talner LB, Santini LC, Lang EK, Gerber FH, Stolberg HO (1988). "Protective effects of corticosteroids in contrast material anaphylaxis". Invest Radiol. 23 Suppl 1: S193–4. doi:10.1097/00004424-198809001-00035. PMID 3058630.
- ↑ Wittbrodt ET, Spinler SA (1994). "Prevention of anaphylactoid reactions in high-risk patients receiving radiographic contrast media". Ann Pharmacother. 28 (2): 236–41. doi:10.1177/106002809402800215. PMID 8173143.
- ↑ Katayama H; Yamaguchi K.; Kozuka T.; et al. (1990). ", "Adverse Reactions to Ionic and Nonionic Contrast Media. A Report from the Japanese Committee on the Safety of Contrast Media". Radiology. 175: 621–28. doi:10.1148/radiology.175.3.2343107.
- ↑ Hamilton Gavin (1990). "Medical Rubber Anaphylaxis". Lancet. 336: 1453. doi:10.1016/0140-6736(90)93165-l.
- ↑ Book review "The Nurses are Innocent," Lethal, odd and ‘new’ in pharmacovigilance. Uppsala Reports 61 - April 2013: Page 10–11.
- ↑ Lasser E.C.; Lyon G.L.; Berry C.C. (1997). "Reports on Contrast Media Reactions: Analysis of Data from Reports to the U.S. Food and Drug Administration". Radiology. 203: 605–10. doi:10.1148/radiology.203.3.9169676.
- ↑ https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-110102
- 1 2 3 Coakley F, Panicek D (1997). "Iodine allergy: an oyster without a pearl?". AJR Am J Roentgenol. 169 (4): 951–2. doi:10.2214/ajr.169.4.9308442. PMID 9308442.
- ↑ Shehadi W (1975). "Adverse reactions to intravascularly administered contrast media. A comprehensive study based on a prospective survey". Am J Roentgenol Radium Ther Nucl Med. 124 (1): 145–52. doi:10.2214/ajr.124.1.145. PMID 1170768.
- ↑ van Ketel W, van den Berg W (1990). "Sensitization to povidone-iodine". Dermatol Clin. 8 (1): 107–9. PMID 2302848.
- ↑ Barrett BJ, Parfrey PS (2006). "Clinical practice. Preventing nephropathy induced by contrast medium". N. Engl. J. Med. 354 (4): 379–86. doi:10.1056/NEJMcp050801. PMID 16436769.
- ↑ https://web.archive.org/web/20080905145600/http://www.amershamhealth-us.com/medpro/clinref/glucophage.html. Archived from the original on September 5, 2008. Retrieved November 7, 2009. Missing or empty
|title=(help) - ↑ Thomsen HS, Morcos SK, and members of the Contrast Media Safety Committee of the European Society of Urogenital Radiology. Contrast media and Metformin. Guidelines to distinguish the risk of lactic acidosis in non-insulin dependent diabetics after administration of contrast media. European Radiology, 1999; 9: 738–740.