Ammonium ferric citrate
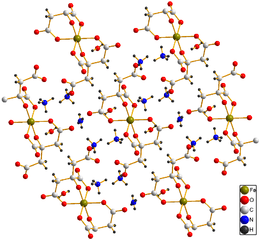 Crystal structure of (NH4)5[Fe(C6H4O7)2]·2H2O[1] | |
| Names | |
|---|---|
| IUPAC name
2-Hydroxypropane-1,2,3-tricarboxylate, ammonium iron(3+) salt | |
| Other names
Ferric ammonium citrate ammonium iron(III) citrate ammonium ferric citrate iron ammonium citrate FerriSeltz | |
| Identifiers | |
| 1185-57-5 | |
| ChEMBL | ChEMBL1200460 |
| ECHA InfoCard | 100.013.351 |
| E number | E381 (antioxidants, ...) |
| |
| Properties | |
| C6H8O7⋅xFe3+⋅yNH3 | |
| Appearance | yellow crystals |
| Pharmacology | |
| V08CA07 (WHO) | |
| Hazards | |
| Safety data sheet | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ammonium ferric citrate has the formula (NH4)5[Fe(C6H4O7)2]. A distinguishing feature of this compound is that it is very soluble in water, in contrast to ferric citrate which is not very soluble.
In its crystal structure each citric acid moiety has lost four protons, and the deprotonated hydroxyl groups act as ligands together with four carboxylate groups; two carboxylate groups are not coordinated to the ferric ion.[1]
Uses
Ammonium ferric citrate has a range of uses, including:
- As a food additive, where it has the E number E381, and is used as an acidity regulator.
- Water purification
- As a reducing agent of metal salts of low activity like gold and silver.
- With potassium ferricyanide as part of the cyanotype photographic process.
- Used in Kligler iron deeps to determine hydrogen sulfide production in microbial metabolism.
- In medical imaging, ammonium ferric citrate is used as a contrast medium.
- As a hematinic.[2]
See also
References
- 1 2 Matzapetakis, M.; Raptopoulou, C. P.; Tsohos, A.; Papaefthymiou, V.; Moon, N.; Salifoglou, A. (1998). "Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2·2H2O". J. Am. Chem. Soc. 120 (50): 13266–13267. doi:10.1021/ja9807035.
- ↑ Budavari, Susan, ed. (2001), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (13th ed.), Merck, ISBN 0911910131
This article is issued from Wikipedia - version of the 11/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.