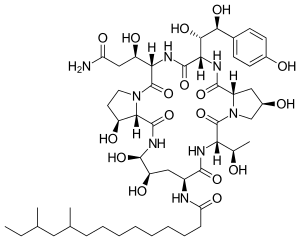
Pneumocandin Bo
 | |
| Names | |
|---|---|
| IUPAC name
N-{(2R,6S,9S,11R,12R,14aS,15S,20S,23S,25aS)-20-[(1S)-3-Amino-1-hydroxy-3-oxopropyl]-23-[(1S,2S)-1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl]-2,11,12,15-tetrahydroxy-6-[(1S)-1-hydroxyethyl]-5,8,14,19,22,25-hexaoxotetracosahydro-1H-dipyrrolo[2,1-c:2',1'-l][1,4,7,10,13,16]hexaazacyclohenicosin-9-yl}-10,12-dimethyltetradecanamide | |
| Other names
HEMBL269311; 135575-42-7; Pneumocandin B0; Hydroxy Echinocandin; SCHEMBL8444763 | |
| Identifiers | |
| 135575-42-7 | |
| 3D model (Jmol) | Interactive image |
| PubChem | 5742645 |
| |
| |
| Properties | |
| C50H80N8O17 | |
| Molar mass | 1065.21 |
| Appearance | White crystalline powder |
| Density | 1.411 g.cm−3 |
| Boiling point | 1,442.936 °C (2,629.285 °F; 1,716.086 K) |
| Soluble in ethanol, methanol, DMF or DMSO. Limited water solubility. | |
| Refractive index (nD) |
1.629 |
| Hazards | |
| Flash point | 826.458 ºC |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
.png)
Pneumocandin Bo, also known as pneumocandinB0, pneumocandin B0, pneumocandin B(0) and hydroxy echinocandin, is an organic chemical compound with formula C50H80N8O17.[1]
It is a strong antifungal and inhibits the synthesis of β-(1→3)-D-glucan, which is a fundamental component in most cell walls, like the Candida albicans membrane. This is a very important activity since there is an increase in the frequency of fungal infections, accompanied by an increase in the variety of opportunistic and pathogenic fungi like Candida.
This compound is used to synthesize caspofungin.[2]
Pneumocandin B0 can be easily confused with pneumocandin B, although they have different kind of chains and residues.
Production
Pneumocandin B0 is the starting molecule for the first semisynthetic echinocandin antifungal drug, caspofungin acetate.
In the wild-type strain, pneumocandin B0 is a minor fermentation product, and its industrial production was achieved by a combination of extensive mutation and medium optimization.
The pneumocandin biosynthetic gene cluster was previously elucidated by a whole genome sequencing approach. Knowledge of the biosynthetic cluster suggested an alternative way to exclusively produce pneumocandin B0.
Disruption of GLOXY4, encoding a nonheme, α-ketoglutarate-dependent oxygenase, confirmed its involvement in L-leucine cyclization to form (4S)-methyl-L-proline. The absence of (4S)-methyl-L-proline abolishes pneumocandin A0 production, and (3S)-hydroxyl-L-proline occupies the hexapeptide core's position 6, resulting in exclusive production of pneumocandin B0.
Retrospective analysis of the GLOXY4 gene in a previously isolated pneumocandin B0-exclusive mutant (ATCC 74030) indicated that chemical mutagenesis disrupted the GLOXY4 gene function by introducing two amino acid mutations in GLOXY4.
This one-step genetic manipulation can rationally engineer a high-yield production strain.[3]
Special features
The echinocandins and pneumocandins are lipopeptides antifungal agents that inhibit the synthesis of β-(1→3)-D-glucan, an essential cell wall homopolysaccharide found in many pathogenic fungi.
Compounds with this fungal-specific target have several attractive features:
- Lack of mechanism-based toxicity
- Potential for fungicidal activity
- Activity against strains with intrinsic or acquired resistance mechanisms for existing antimycotics.[4]
Caspofungin
Caspofungin, a semisynthetic derivate of the pneumocandin B0, is the first licensed compound of a new class of antifungal agent, that are called the echinocandins. This antifungal agent attacks the fungal cell by selective inhibition of β-(1→3)-D-glucan synthase, which is not present in mammalian cells. Caspofungin represents an interesting and clinically valuable new antifungal drug that broadens the available therapeutic armamentarium for the treatment of invasive fungal infections. [5]
Semisynthetic pneumocandin B0 derivatives
The antipneumocystis activities of the pneumocandins can be significantly improved through synthetic modification.
There are new semisynthetic pneumocandin B0 derivatives that have been found:
- By the addition of an aminoethyl ether at the R3 position of pneumocandin B0 resulted water-soluble and nonprodrug compounds, substantially more efficacious
- By the modification of pneumocandin B0 at the R2 position by the conversion of the hydroxyglutamine to a hydroxyornithine increases the antipneumocystis activities of the compounds by 4-fold.
These two modifications combined were synergistic, resulting in a 10-fold improvement in potency against Pneumocystis jirovecii (formerly known as Pneumocystis carinii) pneumonia.[6]
References
- ↑ "Pneumocandin Bo". Pubchem Open Chemistry Database. Retrieved 17 October 2015.
- ↑ Bouffard, FA; Zambias, RA; Dropinski, JF; Balkovec, JM; Hammond, ML; Abruzzo, GK; Bartizal, KF; Marrinan, JA; Kurtz, MB (21 January 1994). "Synthesis and Antifungal Activity of Novel Cationic Pneumocandin Bo Derivatives". Journal of Medicinal Chemistry. 37 (2): 222–5. doi:10.1021/jm00028a003. PMID 8295208.
- ↑ Chen, Li; Yue, Qun; Li, Yan; Niu, Xuemei; Xiang, Meichun; Wang, Wenzhao; Bills, Gerald F.; Liu, Xingzhong; An, Zhiqiang (2015-03-01). "Engineering of Glarea lozoyensis for Exclusive Production of the Pneumocandin B0 Precursor of the Antifungal Drug Caspofungin Acetate". Applied and Environmental Microbiology. 81 (5): 1550–1558. doi:10.1128/AEM.03256-14. ISSN 0099-2240. PMC 4325176
 . PMID 25527531.
. PMID 25527531. - ↑ Kurtz, M. B.; Douglas, C. M. (1997-01-01). "Lipopeptide inhibitors of fungal glucan synthase". Journal of Medical and Veterinary Mycology. 35 (2): 79–86. doi:10.1080/02681219780000961. ISSN 0268-1218. PMID 9147267.
- ↑ Maschmeyer, Georg; Glasmacher, Axel (2005-07-01). "Pharmacological properties and clinical efficacy of a recently licensed systemic antifungal, caspofungin". Mycoses. 48 (4): 227–234. doi:10.1111/j.1439-0507.2005.01131.x. ISSN 1439-0507.
- ↑ "New Semisynthetic Pneumocandins with Improved Efficacies against Pneumocystis carinii in the Rat" (PDF).