Pillararene
Pillararenes are macrocycles composed of hydroquinone units (5 to 10) linked in the para position. They are structurally similar to the cucurbiturils and calixarenes that play an important part in host-guest chemistry. The first pillar[n]arene macrocycle was the five membered derivative, pillar[5]arene.[1][2]

History
1,4-Dimethoxypillar[5]arene (DMpillar[5]arene), the first pillararene, was reported in 2008 Journal of the American Chemical Society by Tomoki Ogoshi et al. Ogoshi et al catalyzed 1,4-dimethoxybenzene by Lewis Acid with paraformaldehyde to obtain 1,4-dimethoxypillar[5]arene. The methoxy groups of DMpillar[5]arene were deprotected using boron tribromide to give pillar[5]arene. Ogoshi named the new family of host macrocycles “pillararene”, since they are cylindrical or "pillar" in shape and composed of aromatic or "arene" moieties.1 Multiple chemists refer to the macrocycle as "pillarene"[3] orally as this is often easier to pronounce and remember.
Structure
Pillararenes are composed of hydroquinone units linked by methylene bridges at para-positions. Its features a symmetrical pillar architecture with two identical cavity gates. Pillar[5]arene is the most conformationally stable member in this family. Due to the close proximity of many electron-rich hydroquinones, the cavity of pillararenes are able to form strong association complexes with electron-poor species. Also, derivatives of the pillararenes can be generated by modifying the hydroxyl groups at all positions or selectively on one or two positions.[4]
Planar chirality
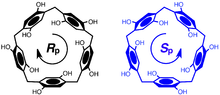
The orientation of the hydroquinone oxygens on both rims of the pillararene allow the macrocycle to exhibit planar chirality. When the substituent on the hydroquinone oxygen is small enough to fit through the cavity of the pillararene, allowing for oxygen-through-the-annulus rotation to occur, racemization occurs. If this substituent is large enough to prevent rotation, optically active pillararene macrocycles can be isolated.[5][6]
A pillar[n]arene macrocycle, with n hydroquinone units, has n planes of chirality. DMpillar[5]arene has five planes of chirality which are "in sync" in the lowest energy conformational isomer due to unfavorable steric interactions between the methoxy groups of neighboring 1,4-dimethoxy-phenylene units. The absolute stereochemical designation of these planes of chirality in pillararene structures can be assigned following modified Cahn-Ingold-Prelog priority rules. The pilot atom for one of the five planes of chirality in pillar[5]arene is assigned to the highest priority atom that is not in the chiral plane - the first carbon atom of the neighboring phenylene unit. The three adjacent in-plane atoms are then assigned, starting with the methylene carbon attached to the pilot atom as priority 1, and the directly connected phenylene carbon as 2, and the carbon atom connected to the methoxy group as 3. When viewed from the side of the pilot atom, if the three atoms form a clockwise direction when followed in order of priority, the molecule is assigned as R(p), otherwise it is assigned as S(p).[7][8]
Synthesis
Homopillararenes
Three strategies are mainly used to obtain pillararenes (Scheme 1).[9] All of three strategies use an acid as catalyst.
- The Lewis acid[10] or Trifluoromethanesulfonic acid catalyzed condensation of 1,4-dialkoxybenzene and paraformaldehyde at room temperature.
- The condensation of 1,4-dialkoxy-2,5bis(alkoxymethyl)benzene catalyzed by p-toluenesulfonic acid.
- Cyclooligomerization of 2,5-dialkoxybenzyl alcohols or 2,5-dialkoxybenzyl bromides with an appropriate Lewis acid as the catalyst.
Copillararenes[11]
In 2010, Feihe Huang et al.[12] introduced three new ways to synthesize copillararenes, which are composed of different repeating units. It is easier to selectively functionalize copillararenes, helping to generate interesting physical properties, conformations, and host-guest binding interactions. There are two possible ways to make copillararenes: to selectively modify repeating monomers of homopillararenes, or to use two different monomers to carry out co-oligomerization.
Mechanism
Pillararenes are traditionally formed through a thermodynamically controlled Friedel-Crafts cyclooligomerization.[13] A practical and effective trifluoromethanesulfonic acid (TfOH)-catalyzed cyclooligomerization strategy was also developed for the synthesis of functionalized pillar[n]arenes and copillar[5]arenes from 1,4-dialkoxybenzenes with paraformaldehyde under mild reaction conditions, and the reaction mechanism of solution-phase catalytic synthesis of pillararenes was investigated by room-temperature X-band ESR spectroscopy, mass spectroscopy, NMR and control experiments, suggesting a free radical process initially and a Friedel–Crafts alkylation process during the consequent coupling and ring-closure stage.[14]
Selective Synthesis of Pillar[6]arene
Pillar[6]arene can be targeted as the major product of the Friedel-Crafts cyclooligomerization by using bulky alkoxy groups on the monomer, switching the Lewis acid catalyst [15] or by using a bulky chlorinated solvent. Ogoshi and coworkers reported [16] the synthesis of a pillar[6]arene with 1,4-Bis(methylcyclohexyl ether)phenylene units in an 87% yield by using chlorocyclohexane as the solvent. The bulky chlorinated solvent was suggested to act as a template for the formation of the larger pillar[n]arene.
Higher Pillar[n]arenes
The higher pillar[n]arene homologues, pillar[6-15]arene, have been synthesized through the ring expansion of pillar[5]arene.[17]
Biomedical applications

While native pillar[n]arenes display no solubility in water, and are therefore unsuitable for biomedical applications, a range of water soluble pillar[n]arenes have been reported. In particular, water soluble carboxylated-pillar[n]arenes (where n = 6 or 7) have already shown potential in both drug delivery and bio-diagnostics because they are highly soluble, form host-guest complexes with a range of drug and medicine-based compounds, and appear to be relatively non-toxic.[18] The cavity of carboxylated-pillar[5]arene is too small to include most drug molecules and therefore is not useful in drug delivery. To form a host-guest complex with the pillar[n]arenes, the drug must have a cationic charge; it's ability to hydrogen-bonds with the pillar[n]arenes is less important. Two planar dye molecules, like proflavine, can be simultaneously encapsulated within the cavity of a single caboxylated-pillar[7]arene. Because encapsulation within the cavity of carboxylated-pillar[6]arene quenches the fluorescence of proflavine, this gives rise to "on" and "off' states to the dye which may have application in bio-diagnostics.
Other potential applications
Pillar[n]arenes have been shown to have potential applications in molecular machinery,[19] sensing, nanoparticle synthesis,[20][21] artificial transmembrane channels,[22] as components in complex, supramolecular controlled drug delivery systems,[23][24] construction of porous materials for gas/guest absorption,[25] [26] organic light-emitting materials, [27] [28] and ionic liquids.
Researchers at Jilin University have reported[29] that a percarboxylated derivative of pillar[5]arene inhibits the assembly of the human papillomavirus.
References
- ↑ Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-a.; Nakamoto, Y., para-bridged symmetrical pillar 5 arenes: Their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130 (15), 5022–5023.
- ↑ Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H.; A Facile and Efficient Preparation of Pillararenes and a Pillarquinone. Angew. Chem. Int. Ed. 2009, 48, 9721-9723.
- ↑ Tan, L.-L.; Zhang, Y.; Li, B.; Wang, K.; Zhang, S. X.-A.; Tao, Y.; Yang, Y.-W. Selective Recognition of “Solvent” Molecules in Solution and the Solid State by 1,4-Dimethoxypillar[5]arene Driven by Attractive Forces. New J. Chem. 2014, 38, 845-851.
- ↑ Strutt, N. L.; Forgan, R. S.; Spruell, J. M.; Botros, Y. Y.; Stoddart, J. F. Monofunctionalized Pillar[5]arene as a Host for Alkanediamines. J. Am. Chem. Soc. 2011, 133, 5668-5671.
- ↑ Ogoshi, T.; Masaki, K.; Shiga, R.; Kitajima, K.; Yamagishi, T.-a. Org. Lett. 2011, 13, 1264
- ↑ Strutt, N. L., Fairen-Jimenez, D.; Iehl, J.; Lalonde, M. B.; Snurr, R. Q.; Farha, O. K.; Hupp, J. T.; Stoddart, J. F. J. Am. Chem. Soc. 2012, 134, 17436.
- ↑ Strutt, N. L., Schneebeli, S. T.; Stoddart, J. F. Stereochemical Inversion in Difunctionalised Pillar[5]arenes. Supramol. Chem., 2013, 25 , 596-608.
- ↑ Ke, C.; Strutt, N. L.; Li, H.; Hou, X.; Hartlieb, K.; McGonigal, P. R.; Ma, Z.; Iehl, J.; Stern, C. L.; Cheng, C.; Zhu, Z.; Vermeulen, N. A.; Meade, T. J.; Botros, Y. Y.; Stoddart, J. F. Pillar[5]arene as a Co-Factor in Templating Rotaxane Formation. J. Am. Chem. Soc. 2013, 135, 17019-17030.
- ↑ Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F., Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45 (8), 1294–1308.
- ↑ Liu, L.; Cao, D.; Jin, Y.; Tao, H.; Kou, Y.; Meier, H., Efficient Synthesis of Copillar[5]Arenes and Their Host-Guest Properties with Dibromoalkanes. Org. Biomol. Chem. 2011, 9, 7007-7010.
- ↑ Liu, L.; Cao, D.; Jin, Y.; Tao, H.; Kou, Y.; Meier, H., Efficient Synthesis of Copillar[5]Arenes and Their Host-Guest Properties with Dibromoalkanes. Org. Biomol. Chem. 2011, 9, 7007-7010.
- ↑ Zhang, Z.; Xia, B.; Han, C.; Yu, Y.; Huang, F. Syntheses of Copillar[5]arenes by Co-oligomerization of Different Monomers, Organic Letters, 2010, 12, 3285-3287.
- ↑ Holler, M.; Allenbach, N.; Sonet, J.; Nierengarten, J.-F. The high yielding synthesis of pillar[5]arenes under Friedel-Crafts conditions explained by dynamic covalent bond formation. Chem. Commun. 2012, 48, 2576.
- ↑ Wang, K.; Tan, L.-L.; Chen, D.-X.; Song, N.; Xi, G.; Zhang, S. X.-A.; Li, C.; Yang, Y.-W. One-Pot Synthesis of Pillar[n]arenes Catalyzed by a Minimum Amount of TfOH and a Solution-Phase Mechanistic Study. Org. Biomol. Chem. 2012, 10, 9405-9409.
- ↑ Synthesis and host-guest properties of pillar[6]arenes. Sci. China Chem. 2012, 55, 223-228.
- ↑ The template effect of solvents on high yield synthesis, co-cyclization of pillar[6]arenes and interconversion between pillar[5]- and pillar[6]arenes. Chem. Commun. 2014, 50, 5774-5777.
- ↑ Tomoki Ogoshi, Naosuke Ueshima, Fumiyasu Sakakibara, Tada-aki Yamagishi, and Takeharu Haino. Conversion from Pillar[5]arene to Pillar[6–15]arenes by Ring Expansion and Encapsulation of C60 by Pillar[n]arenes with Nanosize Cavities. Org. Lett. 2014, 16, 2896-2899. DOI: 10.1021/ol501039u.
- ↑ Wheate, Nial; Dickson, Kristie-Ann; Kim, Ryung Rae; Nematollahi, Alireza; Macquart, Rene; Kayser, Veysel; Yu, Guocan; Church, W. Bret; Marsh, Deborah (2016). "Host-Guest Complexes of Carboxylated Pillar[n]arenes With Drugs". Journal of Pharmaceutical Science. 105: 3615–3625. doi:10.1016/j.xphs.2016.09.008.
- ↑ Hou, X.; Ke, C.; Cheng, C.; Song, N.; Blackburn, A. K.; Sarjeant, A. A.; Botros, Y. Y.; Yang, Y.-W.; Stoddart, J. F. Efficient Syntheses of Pillar[6]arene-Based Hetero[4]rotaxanes Using a Cooperative Capture Strategy. Chem. Commun. 2014, 50, 6196-6199.
- ↑ Li, H.; Chen, D.-X.; Sun, Y.-L.; Zheng, Y. B.; Tan, L.-L.; Weiss, P. S.; Yang, Y.-W. Viologen-Mediated Assembly of and Sensing with Carboxylatopillar[5]arene-Modified Au Nanoparticles. J. Am. Chem. Soc. 2013, 135, 1570-1576.
- ↑ Yao, Y.; Xue, M.; Chi, X.; Ma, Y.; He, J.; Abliz, Z.; Huang, F. A New Water-Soluble Pillar[5]arene: Synthesis and Application in the Preparation of Gold Nanoparticles. Chem. Commun. 2012, 48, 6505−6507.
- ↑ Si, W.; Chen, L.; Hu, X.-B.; Tang, G.; Chen, Z.; Hou, J.-L.; Li, Z.-T. Selective Artificial Transmembrane Channels for Protons by Formation of Water Wires. Angew. Chem. Int. Ed. 2011, 50, 12564–12568.
- ↑ Sun, Y.-L.; Yang, Y.-W.; Chen, D.-X.; Wang, G.; Zhou, Y.; Wang, C.-Y.; Stoddart, J. F. Mechanized Silica Nanoparticles Based on Pillar[5]arenes for On-Command Cargo Release. Small 2013, 9, 3224-3229.
- ↑ Duan, Q.; Cao, Y.; Li, Y.; Hu, X.; Xiao, T.; Lin, C.; Pan, Y.; Wang, L. pH-Responsive Supramolecular Vesicles Based on Water-Soluble Pillar[6]arene and Ferrocene Derivative for Drug Delivery. J. Am. Chem. Soc. 2013, 135, 10542–10549.
- ↑ Strutt, N. L.; Fairen-Jimenez, D.; Iehl, J.; LaLonde, M. B.; Snurr, R. Q.; Farha, O. K.; Hupp, J. T.; Stoddart, J. F. Incorporation of an A1/A2-difunctionalized pillar[5]arene into a metal–organic framework. J. Am. Chem. Soc. 2012, 134, 17436—17439
- ↑ Tan, L.-L.; Li, H.; Tao, Y.; Zhang, S. X.-A.; Wang, B.; Yang, Y.-W. Pillarene-Based Supramolecular Organic Frameworks for Highly Selective Carbon Dioxide Storage under Ambient Conditions. Adv. Mater. 2014, 26, 7027–7031.
- ↑ Song, N.; Chen, D.-X.; Qiu, Y.-C.; Yang, X.-Y.; Xu, B.; Tian, W.; Yang, Y.-W. Stimuli-Responsive Blue Fluorescent Supramolecular Polymers Based on a Pillar[5]arene Tetramer. Chem. Commun. 2014, 50, 8231–8234.
- ↑ Song, N.; Chen, D.-X.; Xia, M.-C.; Qiu, X.-L.; Ma, K.; Xu, B.; Tian, W.; Yang, Y.-W. Supramolecular Assembly-Induced Yellow Emission of 9,10-Distyrylanthracene Bridged Bis(pillar[5]arene)s. Chem. Commun. 2014, DOI: 10.1039/C4CC08205B.
- ↑ Zheng, D.-D.; Fu, D.-Y.; Wu, Y.-Q.;* Sun, Y.-L.; Tan, L.-L.; Zhou, T.; Ma, S.-Q.; Zha, X.; Yang, Y.-W. Efficient Inhibition of Human Papillomavirus 16 L1 Pentamer Formation by a Carboxylatopillarene and a p-Sulfonatocalixarene. Chem. Commun. 2014, 50, 3201-3203.