Hofmann rearrangement
| Hofmann rearrangement | |
|---|---|
| Named after | August Wilhelm von Hofmann |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000410 |
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atoms.[1][2][3]
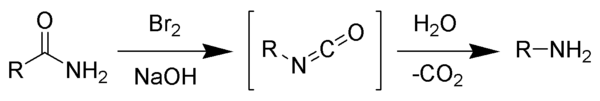
The reaction is named after its discoverer - August Wilhelm von Hofmann. This reaction is also sometimes called the Hofmann degradation or the Harmon Process, and should not be confused with the Hofmann elimination.
Mechanism
The reaction of bromine with sodium hydroxide forms sodium hypobromite in situ, which transforms the primary amide into an intermediate isocyanate. The formation of an intermediate nitrene is not possibile because it implies also the formation of an hydroxamic acid as a byproduct, which has never been observed. The intermediate isocyanate is hydrolyzed to a primary amine, giving off carbon dioxide.[2]
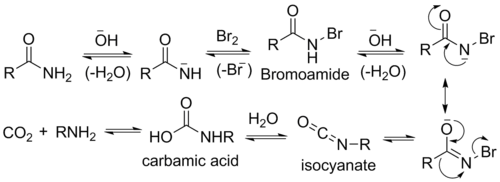
- Base abstracts an acidic N-H proton, yielding an anion.
- The anion reacts with bromine in an α-substitution reaction to give an N-bromoamide.
- Base abstraction of the remaining amide proton gives a bromoamide anion.
- The bromoamide anion rearranges as the R group attached to the carbonyl carbon migrates to nitrogen at the same time the bromide ion leaves, giving an isocyanate.
- The isocyanate adds water in a nucleophilic addition step to yield a carbamic acid (aka urethane).
- The carbamic acid spontaneously loses CO2, yielding the amine product.
Variations
Several reagents can substitute for bromine. Sodium hypochlorite,[4] Lead tetraacetate,[5] N-bromosuccinimide, (bis(trifluoroacetoxy)iodo)benzene,[6] and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) can affect a Hofmann rearrangement. In the following example, the intermediate isocyanate is trapped by methanol, forming a carbamate.[7]

In a similar fashion, the intermediate isocyanate can be trapped by tert-butyl alcohol, yielding the tert-butoxycarbonyl (Boc)-protected amine.
The Hofmann Rearrangement also can be used to yield carbamates from α,β-unsaturated or α-hydroxy amides[2][8] or nitriles from α,β-Acetylenic amides[2][9] in good yields (≈70%).
For Amiloride, hypobromous acid was used to effect Hofmann rearrangement.
Applications
- Aliphatic & Aromatic amides are converted into aliphatic and aromatic amines, respectively
- In the preparations of anthranilic acid from phthalimide
- Nicotinic acid is converted into 3-Amino pyridine
- The Symmetrical structure of α-phenyl propanamide does not change after Hofmann reaction.
- Gabapentin from mono-amidation 1,1-cyclohexane diacetic acid anhydride with ammonia to 1,1-cyclohexane diacetic acid mono-amide; followed by ‘Hoffmann’ rearrangement: U.S. Patent 20,080,103,334
See also
- Beckmann rearrangement
- Curtius rearrangement
- Iodoform reaction
- Lossen rearrangement
- Schmidt reaction
- Weerman degradation
References
- ↑ Hofmann, A. W. (1881). "Ueber die Einwirkung des Broms in alkalischer Lösung auf Amide" [On the action of bromine in alkaline solution on amides]. Berichte der deutschen chemischen Gesellschaft. 14 (2): 2725–2736. doi:10.1002/cber.188101402242.
- 1 2 3 4 Everett, Wallis; Lane, John (1946). "The Hofmann Reaction". Organic Reactions. 3 (7): 267–306. doi:10.1002/0471264180.or003.07. ISBN 9780471005285.
- ↑ Shioiri, Takayuki (1991). "Degradation Reactions". Comprehensive Organic Synthesis. 6: 795–828. doi:10.1016/B978-0-08-052349-1.00172-4. ISBN 9780080359298.
- ↑ Mohan, Ram S.; Monk, Keith A. (1999). "The Hofmann Rearrangement Using Household Bleach: Synthesis of 3-Nitroaniline". Journal of Chemical Education. 76 (12): 1717. doi:10.1021/ed076p1717.
- ↑ Baumgarten, Henry; Smith, Howard; Staklis, Andris (1975). "Reactions of amines. XVIII. Oxidative rearrangement of amides with lead tetraacetate". The Journal of Organic Chemistry. 40 (24): 3554–3561. doi:10.1021/jo00912a019.
- ↑ Almond, Merrick R.; Stimmel, Julie B.; Thompson, Alan; Loudon, Marc (1988). "Hofmann Rearrangement under Mildly Acidic Conditions using [I,I-Bis(Trifluoroacetoxy)]iodobenzene: Cyclobutylamine Hydrochloride from Cyclobutanecarboxamide". Organic Syntheses. 66: 132. doi:10.15227/orgsyn.066.0132.
- ↑ Keillor, Jeffrey W.; Huang, Xicai (2002). "Methyl Carbamate Formation via Modified Hofmann Rearrangement Reactions: Methyl N-(p-Methoxyphenyl)carbamate". Organic Syntheses. 78: 234. doi:10.15227/orgsyn.078.0234.
- ↑ Weerman, R.A. (1913). "Einwirkung von Natriumhypochlorit auf Amide ungesättigter Säuren". Justus Liebigs Annalen der Chemie. 401 (1): 1–20. doi:10.1002/jlac.19134010102.
- ↑ Rinkes, I. J. (1920). "De l'action de l'Hypochlorite de Sodium sur les Amides D'Acides". Recueil des Travaux Chimiques des Pays-Bas. 39 (12): 704–710. doi:10.1002/recl.19200391204.
Bibliography
- Clayden, Jonathan (2007). Organic Chemistry. Oxford University Press Inc. p. 1073. ISBN 978-0-19-850346-0.
- Fieser, Louis F. (1962). Advanced Organic Chemistry. Reinhold Publishing Corporation, Chapman & Hall, Ltd. pp. 499–501.