Schmidt reaction
| Schmidt reaction | |
|---|---|
| Named after | Karl Friedrich Schmidt |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| Organic Chemistry Portal | schmidt-reaction |
| RSC ontology ID | RXNO:0000170 |
The Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl group to give an amine or amide, with expulsion of nitrogen.[1][2][3] It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide.[4] Surprisingly, the intramolecular reaction wasn't reported until 1991[5] but has become important in the synthesis of natural products[6]
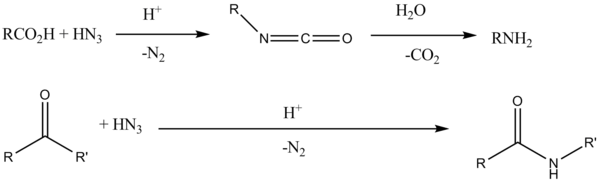
Reaction mechanism
The carboxylic acid Schmidt reaction starts with acylium ion 1 obtained from protonation and loss of water. Reaction with hydrazoic acid forms the protonated azido ketone 2, which goes through a rearrangement reaction with the alkyl group R, migrating over the C-N bond with expulsion of nitrogen. The protonated isocyanate is attacked by water forming carbamate 4, which after deprotonation loses carbon dioxide to the amine.
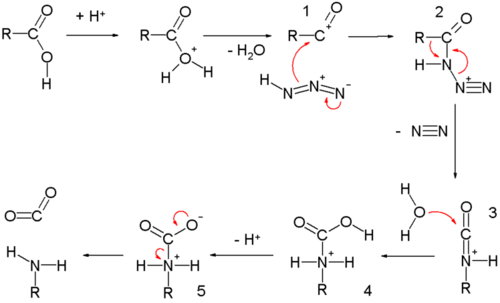
The reaction is related to the Curtius rearrangement except that in this reaction the azide is protonated and hence with different intermediates.
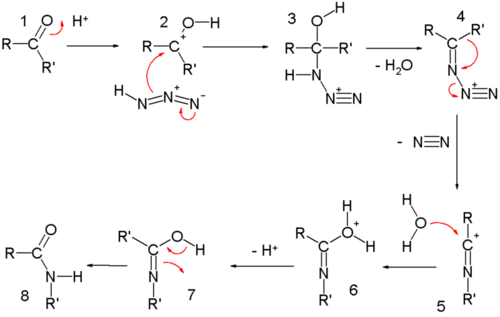
In the reaction mechanism for the ketone Schmidt reaction, the carbonyl group is activated by protonation for nucleophilic addition by the azide, forming intermediate 3, which loses water in an elimination reaction to temporary imine 4, over which one of the alkyl groups migrates from carbon to nitrogen with loss of nitrogen. A similar migration is found in the Beckmann rearrangement. Attack by water and proton loss converts 5 to 7, which is a tautomer of the final amide.
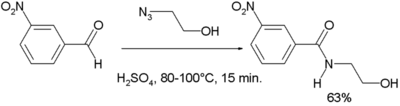
Reactions involving alkyl azides
The scope of this reaction has been extended to reactions of carbonyls with alkyl azides R-N3. This extension was first reported by J.H. Boyer in 1955 [7] (hence the name Boyer reaction), for example, the reaction of m-nitrobenzaldehyde with β-azido-ethanol:
Variations involving intramolecular Schmidt reactions have been known since 1991.[5] These are annulation reactions and have some utility in the synthesis of natural products;[6][8] such as lactams[9] and alkaloids.[10]
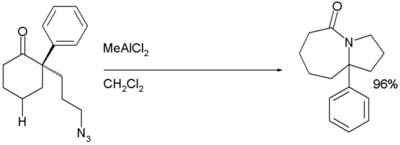
See also
References
- ↑ Plagens, Andreas; Laue, Thomas M. (2005). Named organic reactions (2nd ed.). Chichester: John Wiley & Sons. ISBN 0-470-01041-X.
- ↑ Wolff, Hans (2011). "The Schmidt Reaction": 307–336. doi:10.1002/0471264180.or003.08.
- ↑ Lang, S.; Murphy, J. A. (2006). "Azide rearrangements in electron-deficient systems". Chem. Soc. Rev. 35 (2): 146–156. doi:10.1039/B505080D.
- ↑ Schmidt, K. F. (1924). "Über den Imin-Rest". Berichte der deutschen chemischen Gesellschaft (A and B Series). 57 (4): 704–723. doi:10.1002/cber.19240570423.
- 1 2 Jeffrey Aube & Gregory L. Milligan (1991). "Intramolecular Schmidt reaction of alkyl azides". J. Am. Chem. Soc. 113 (23): 8965–8966. doi:10.1021/ja00023a065.
- 1 2 Nyfeler, Erich; Renaud, Philippe (24 May 2006). "Intramolecular Schmidt Reaction: Applications in Natural Product Synthesis". CHIMIA International Journal for Chemistry. 60 (5): 276–284. doi:10.2533/000942906777674714.
- ↑ J. H. Boyer & J. Hamer (1955). "The Acid-catalyzed Reaction of Alkyl Azides upon Carbonyl Compounds". J. Am. Chem. Soc. 77 (4): 951–954. doi:10.1021/ja01609a045.
- ↑ Milligan, Gregory L.; Mossman, Craig J.; Aube, Jeffrey (October 1995). "Intramolecular Schmidt Reactions of Alkyl Azides with Ketones: Scope and Stereochemical Studies". Journal of the American Chemical Society. 117 (42): 10449–10459. doi:10.1021/ja00147a006.
- ↑ Lei Yao & Jeffrey Aubé (2007). "Cation–π Control of Regiochemistry of Intramolecular Schmidt Reactions en Route to Bridged Bicyclic Lactams" (Communication). J. Am. Chem. Soc. 129 (10): 2766–2767. doi:10.1021/ja068919r. PMC 2596723
 . PMID 17302421.
. PMID 17302421. - ↑ Wrobleski, Aaron; Sahasrabudhe, Kiran; Aubé, Jeffrey (May 2004). "Asymmetric Total Synthesis of Dendrobatid Alkaloids: Preparation of Indolizidine 251F and Its 3-Desmethyl Analogue Using an Intramolecular Schmidt Reaction Strategy". Journal of the American Chemical Society. 126 (17): 5475–5481. doi:10.1021/ja0320018.