H1 antagonist
H1 antagonists, also called H1 blockers, are a class of medications that block the action of histamine at the H1 receptor, helping relieve allergic reactions. Agents where the main therapeutic effect is mediated by negative modulation of histamine receptors are termed antihistamines; other agents may have antihistaminergic action but are not true antihistamines.
In common use, the term "antihistamine" refers only to H1 antagonists, also known as H1-receptor antagonists and H1-antihistamines. It has been discovered that some H1-antihistamines function as inverse agonists, as opposed to receptor antagonists, at the histamine H1-receptor.[1]
Pharmacology
In type I hypersensitivity allergic reactions, an allergen (a type of antigen) interacts with and cross-links surface IgE antibodies on mast cells and basophils. Once the mast cell-antibody-antigen complex is formed, a complex series of events occurs that eventually leads to cell degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Once released, the histamine can react with local or widespread tissues through histamine receptors.
Histamine, acting on H1-receptors, produces pruritus, vasodilation, hypotension, flushing, headache, tachycardia, bronchoconstriction, increase in vascular permeability and potentiation of pain.[2]
While H1-antihistamines help against these effects, they work only if taken before contact with the allergen. In severe allergies, such as anaphylaxis or angioedema, these effects may be of life-threatening severity. Additional administration of epinephrine, often in the form of an autoinjector (Epi-pen), is required by people with such hypersensitivities.
Clinical use of H1-antihistamines
Indications
H1-antihistamines are clinically used in the treatment of histamine-mediated allergic conditions. These indications may include:[3]
- Allergic rhinitis
- Allergic conjunctivitis
- Allergic dermatological conditions (contact dermatitis)
- Rhinorrhea (Runny nose)
- Urticaria
- Angioedema
- Diarrhea
- Pruritus (atopic dermatitis, insect bites)
- Anaphylactic or anaphylactoid reactions—adjunct only
- Nausea and vomiting
- Sedation (first-generation H1-antihistamines)
H1-antihistamines can be administered topically (through the skin, nose, or eyes) or systemically, based on the nature of the allergic condition.
The authors of the American College of Chest Physicians Updates on Cough Guidelines (2006) recommend that, for cough associated with the common cold, first-generation antihistamine-decongestants are more effective than newer, non-sedating antihistamines. First-generation antihistamines include diphenhydramine (Benadryl), carbinoxamine (Clistin), clemastine (Tavist), chlorpheniramine (Chlor-Trimeton), and brompheniramine (Dimetane). However, a 1955 study of "antihistaminic drugs for colds," carried out by the U.S. Army Medical Corps, reported that "there was no significant difference in the proportion of cures reported by patients receiving oral antihistaminic drugs and those receiving oral placebos. Furthermore, essentially the same proportion of patients reported no benefit from either type of treatment."[4]
Adverse drug reactions
Adverse drug reactions are most commonly associated with the first-generation H1-antihistamines. This is due to their relative lack of selectivity for the H1-receptor and their ability to cross the blood-brain barrier.
The most common adverse effect is sedation; this "side-effect" is utilized in many OTC sleeping-aid preparations. Other common adverse effects in first-generation H1-antihistamines include dizziness, tinnitus, blurred vision, euphoria, uncoordination, anxiety, increased appetite leading to weight gain, insomnia, tremor, nausea and vomiting, constipation, diarrhea, dry mouth, and dry cough. Infrequent adverse effects include urinary retention, palpitations, hypotension, headache, hallucination, and psychosis.[3]
The newer, second-generation H1-antihistamines are far more selective for peripheral histamine H1-receptors and have a better tolerability profile compared to the first-generation agents. The most common adverse effects noted for second-generation agents include drowsiness, fatigue, headache, nausea and dry mouth.[3]
First-generation (non-selective, classical)
These are the oldest H1-antihistaminergic drugs and are relatively inexpensive and widely available. They are effective in the relief of allergic symptoms, but are typically moderately to highly potent muscarinic acetylcholine receptor (anticholinergic) antagonists as well. These agents also commonly have action at α-adrenergic receptors and/or 5-HT receptors. This lack of receptor selectivity is the basis of the poor tolerability profile of some of these agents, especially when compared with the second-generation H1-antihistamines. Patient response and occurrence of adverse drug reactions vary greatly between classes and between agents within classes.
Classes
The first H1-antihistamine discovered was piperoxan, by Ernest Fourneau and Daniel Bovet (1933) in their efforts to develop a guinea pig animal model for anaphylaxis at the Pasteur Institute in Paris.[5] Bovet went on to win the 1957 Nobel Prize in Physiology or Medicine for his contribution. Following their discovery, the first-generation H1-antihistamines were developed in the following decades. They can be classified on the basis of chemical structure, and agents within these groups have similar properties.
| Class | Description | Examples |
| Ethylenediamines | Ethylenediamines were the first group of clinically effective H1-antihistamines developed. |
|
| Ethanolamines | Diphenhydramine was the prototypical agent in this group. Significant anticholinergic adverse effects, as well as sedation, are observed in this group but the incidence of gastrointestinal adverse effects is relatively low.[3][6] | |
| Alkylamines | The isomerism is a significant factor in the activity of the agents in this group. E-triprolidine, for example, is 1000-fold more potent than Z-triprolidine. This difference relates to the positioning and fit of the molecules in the histamine H1-receptor binding site.[6] Alkylamines are considered to have relatively fewer sedative and gastrointestinal adverse effects, but relatively greater incidence of paradoxical central nervous system (CNS) stimulation.[3] |
|
| Piperazines | These compounds are structurally related to the ethylenediamines and the ethanolamines, and produce significant anticholinergic adverse effects. Compounds from this group are often used for motion sickness, vertigo, nausea, and vomiting. The second-generation H1-antihistamine cetirizine also belongs to this chemical group.[6] | |
| Tricyclics and Tetracyclics | These compounds differ from the phenothiazine antipsychotics in the ring-substitution and chain characteristics.[6] They are also structurally related to the tricyclic antidepressants (and tetracyclics), explaining the H1-antihistaminergic adverse effects of those three drug classes and also the poor tolerability profile of tricyclic H1-antihistamines. The second-generation H1-antihistamine loratadine was derived from compounds in this group. |
|
Common structural features
- Two aromatic rings, connected to a central carbon, nitrogen or CO
- Spacer between the central X and the amine, usually 2–3 carbons in length, linear, ring, branched, saturated or unsaturated
- Amine is substituted with small alkyl groups, e.g., CH3
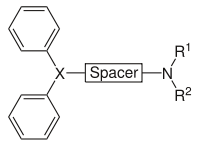
X = N, R1 = R2 = small alkyl groups
X = C
X = CO
- Chirality at X can increase both the potency and selectivity for H1-receptors
- For maximum potency, the two aromatic rings should be orientated in different planes
- for example, tricyclic ring system is slightly puckered and the two aromatic rings lie in different geometrical planes, giving the drug a very high potency.
Second-generation and third-generation (selective, non-sedating)
Second-generation
Second-generation H1-antihistamines are newer drugs that are much more selective for peripheral H1 receptors as opposed to the central nervous system H1 receptors and cholinergic receptors. This selectivity significantly reduces the occurrence of adverse drug reactions, such as sedation, while still providing effective relief of allergic conditions. The reason for their peripheral selectivity is that most of these compounds are zwitterionic at physiological pH (around pH 7.4). As such, they are very polar, meaning that they do not cross the blood–brain barrier and act mainly outside the central nervous system.
- Examples -
Systemic:
- Astemizole
- Ketotifen
- Cetirizine
- Loratadine
- Rupatadine
- Mizolastine
- Acrivastine
- Ebastine
- Bilastine
- Bepotastine
- Terfenadine
- Quifenadine
Topical:
Third-generation
Third-generation H1-antihistamines are second-generation antihistamines informally labeled third-generation because the active enantiomer (levocetirizine) or metabolite (desloratadine & fexofenadine) derivatives of second-generation drugs are intended to have increased efficacy with fewer adverse drug reactions. Fexofenadine is associated with a decreased risk of cardiac arrhythmia compared to terfenadine. However, there is little evidence for any advantage of levocetirizine or desloratadine, compared to cetirizine or loratadine, respectively.
There is some controversy associated with the use of the term third-generation antihistamines.[7]
- Examples -
Systemic:
References
- ↑ Leurs R, Church MK, Taglialatela M (April 2002). "H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects". Clinical & Experimental Allergy. 32 (4): 489–98. doi:10.1046/j.0954-7894.2002.01314.x. PMID 11972592.
- ↑ Simons, F. Estelle R. (November 2004). "Advances in H1-antihistamines". The New England Journal of Medicine. 351 (21): 2203–17. doi:10.1056/NEJMra033121. ISSN 0028-4793. PMID 15548781.
- 1 2 3 4 5 Rossi S (Ed.) (2004). Australian Medicines Handbook 2004. Adelaide: Australian Medicines Handbook. ISBN 0-9578521-4-2
- ↑ Hoagland, RJ; Deitz, EN; Myers, PW; Cosand, HC (May 1950). "Antihistaminic drugs for colds: evaluation based on a controlled study". Journal of the American Medical Association. 143 (2): 157–60. doi:10.1001/jama.1950.02910370007003. ISSN 0002-9955. PMID 15415236.
- ↑ Fourneau, Ernest; Daniel Bovet (1933). "Recherches sur l'action sympathicolytique d'un nouveau dérivé du dioxane". Archives Internationales de Pharmacodynamie et de Thérapie. 46: 178–91. ISSN 0003-9780.
- 1 2 3 4 Nelson, Wendel L. (2007). "Antihistamines and Related Antiallergic and Antiulcer Agents". In William O. Foye, Thomas L. Lemke and David A. Williams. Foye's Principles of Medicinal Chemistry. Hagerstown, Maryland: Lippincott Williams & Wilkins. pp. 1004–1027. ISBN 978-0-7817-6879-5. OCLC 149596645.
- ↑ Camelo-Nunes, Inês Cristina (November 2006). "Novos anti-histamínicos: uma visão crítica (New antihistamines: a critical view)". Jornal de Pediatria (in Portuguese). 82 (5): S173–80. doi:10.1590/S0021-75572006000700007. ISSN 0021-7557. PMID 17136293.
- ↑ Nettis, E; Colanardi, MC; Barra, L; Ferrannini, A; Vacca, A; Tursi, A (March 2006). "Levocetirizine in the treatment of chronic idiopathic urticaria: a randomized, double-blind, placebo-controlled study". The British journal of dermatology. 154 (3): 533–8. doi:10.1111/j.1365-2133.2005.07049.x. ISSN 0007-0963. PMID 16445787.
- ↑ Howell, G, III; West, L; Jenkins, C; Lineberry, B; Yokum, D; Rockhold, R (August 2005). "In vivo antimuscarinic actions of the third generation antihistaminergic agent, desloratadine" (Free full text). BMC pharmacology. 5: 13. doi:10.1186/1471-2210-5-13. PMC 1192807
 . PMID 16109168.
. PMID 16109168. - ↑ Vena, GA; Cassano, N; Filieri, M; Filotico, R; D'Argento, V; Coviello, C (September 2002). "Fexofenadine in chronic idiopathic urticaria: a clinical and immunohistochemical evaluation". International Journal of Immunopathology and Pharmacology. 15 (3): 217–224. ISSN 0394-6320. PMID 12575922.
External links
- Antihistaminics, H1 at the US National Library of Medicine Medical Subject Headings (MeSH)