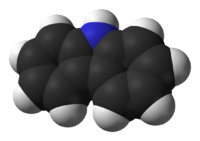
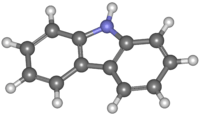
Carbazole
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
9H-carbazole | |
| Other names
9-azafluorene, dibenzopyrrole, diphenylenimine, diphenyleneimide, USAF EK-600 | |
| Identifiers | |
| 86-74-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27543 |
| ChEMBL | ChEMBL243580 |
| ChemSpider | 6593 |
| DrugBank | DB07301 |
| ECHA InfoCard | 100.001.542 |
| KEGG | C08060 |
| PubChem | 6854 |
| UNII | 0P2197HHHN |
| |
| |
| Properties | |
| C12H9N | |
| Molar mass | 167.206 g mol−1[1] |
| Density | 1.301g/cm^3 |
| Melting point | 246.3 °C (475.3 °F; 519.5 K)[1] |
| Boiling point | 354.69 °C (670.44 °F; 627.84 K)[1] |
| Hazards | |
| Flash point | 220 °C (428 °F; 493 K) [1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole (equivalent to the 9a–4a double bond in carbazole respectively).
Synthesis
A classic laboratory organic synthesis for carbazole is the Borsche–Drechsel cyclization.[2][3]
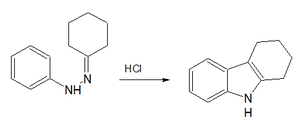 Borsche–Drechsel synthesis
Borsche–Drechsel synthesis
In the first step phenylhydrazine is condensed with cyclohexanone to the corresponding imine. The second step is a hydrochloric acid catalyzed rearrangement reaction and ring-closing reaction to tetrahydrocarbazole. In one modification, both steps are rolled into one by carrying out the reaction in acetic acid.[4] In the third step this compound is oxidized by Red lead to carbazole itself. Another classic is the Bucherer carbazole synthesis
A second method for the synthesis of carbazole is the Graebe–Ullmann reaction.
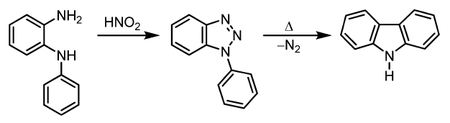
In the first step, an N-phenyl-1,2-diaminobenzene (N-phenyl-o-phenylenediamine) is converted into a diazonium salt which instantaneously forms a 1,2,3-triazole. The triazole is unstable and at elevated temperatures nitrogen is set free and the carbazole is formed.[5][6]
Use
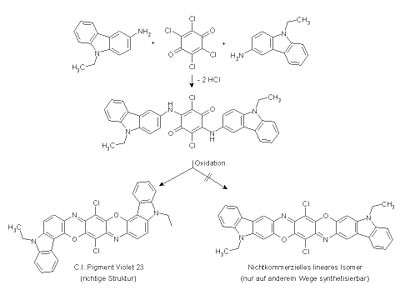
Aminoethylcarbazole is used in the production of pigment violet 23.
- Rimcazole is also made from carbazole proper.
Related aromatic compounds
References
- 1 2 3 4 Lide, David R. (2007). CRC Handbook of Chemistry and Physics, 88th Edition. CRC Press. pp. 3–86. ISBN 978-0-8493-0488-0.
- ↑ W. Borsche (1908). "Ueber Tetra- und Hexahydrocarbazolverbindungen und eine neue Carbazolsynthese. (Mitbearbeitet von. A. Witte und W. Bothe.)". Justus Liebig's Annalen der Chemie. 359 (1–2): 49–80. doi:10.1002/jlac.19083590103.
- ↑ E. Drechsel (1888). "Ueber Elektrolyse des Phenols mit Wechselströmen". Journal für praktische Chemie (in German). 38 (1): 65–74. doi:10.1002/prac.18880380105.
- ↑ Organic Syntheses, Coll. Vol. 4, p.884 (1963); Vol. 30, p.90 (1950). Link
- ↑ Carl Graebe and Fritz Ullmann (1896). "Ueber eine neue Carbazolsynthese". Justus Liebig's Annalen der Chemie. 291 (1): 16–17. doi:10.1002/jlac.18962910104.
- ↑ O. Bremer (1934). "Über die Bedeutung der Graebe-Ullmannschen Carbazolsynthese und deren Übertragung auf N-substituierte Pyridino-triazole". Justus Liebigs Annalen der Chemie. 514: 279–291. doi:10.1002/jlac.19345140116.
External links
| Wikisource has the text of the 1911 Encyclopædia Britannica article Carbazol. |