Pigment violet 23
 | |
| Names | |
|---|---|
| Other names
9,19-Dichloro-5,15-diethyl-5,15-dihydrodiindolo[2,3-c:2',3'-n]triphenodioxazine, C.I. 51319, Carbazole Dioxazine Violet | |
| Identifiers | |
| 215247-95-3 | |
| EC Number | 606-790-9 |
| Properties | |
| C34H22Cl2N4O2 | |
| Molar mass | 589.47 |
| Appearance | dark purple solid |
| Melting point | 385 ºC |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
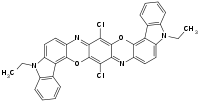
Pigment violet 23 is an organic compound that is a commercial pigment. It is member of the dioxazine family of heterocyclic compounds, but derived from carbazoles.[1] It is prepared by condensation of chloranil and 3-amino-N-ethylcarbazole. It has a centrosymmetric angular structure.[2] For many years, the structure was assigned, incorrectly, as having a "linear structure" (EC no. 228-767-9, CAS RN 6358-30-1) which differ in terms of the carbazole ring fusion.[3]
Pigment violet 23 is prepared by condensation of an amino with chloranil.[4]
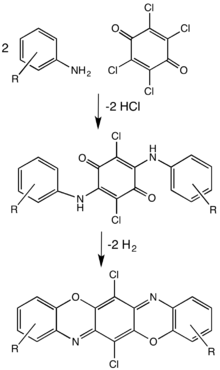
Synthetic route to dioxazine dyes such as pigment violet 23.
References
- ↑ Chamberlain, Terence "Dioxazine violet pigments" from High Performance Pigments Edited by Smith, Hugh M. 2002, 185–194. doi:10.1002/3527600493.ch12
- ↑ Panina, N.; van de Ven, R.; Verwer, P.; Meekes, H.; Vlieg, E.; Deroover, G. "Polymorph prediction of organic pigments" Dyes and Pigments 2008, volume 79, 183–192.
- ↑ Heinrich Zollinger (2003). Color Chemistry Syntheses, Properties, and Applications of Organic Dyes and Pigments. John Wiley & Sons. p. 118. ISBN 3-906390-23-3.
- ↑ Horst Tappe, Walter Helmling, Peter Mischke, Karl Rebsamen, Uwe Reiher, Werner Russ, Ludwig Schläfer and Petra Vermehren "Reactive Dyes"in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_651
This article is issued from Wikipedia - version of the 10/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.