Ferrier carbocyclization
The Ferrier carbocyclization (or Ferrier II reaction) is an organic reaction that was first reported by the carbohydrate chemist Robert J. Ferrier in 1979.[1][2] It is a metal-mediated rearrangement of enol ether pyrans to cyclohexanones. Typically, this reaction is catalyzed by mercury salts, specifically mercury(II) chloride.
Several reviews have been published.[3][4]
Reaction mechanism
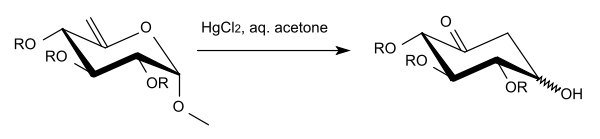
Ferrier proposed the following reaction mechanism:
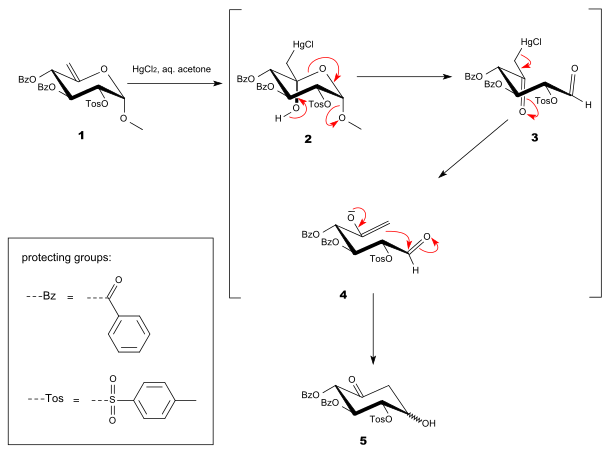
In this mechanism, the terminal olefin undergoes hydroxymercuration to produce the first intermediate, compound 2, a hemiacetal. Next, methanol is lost and the dicarbonyl compound cyclizes through an attack on the electrophilic aldehyde to form the carbocycle as the product. A downside to this reaction is that the loss of CH3OH at the anomeric position (carbon-1) results in a mixture of α- and β-anomers. The reaction also works for substituted alkenes (e. g. having an -OAc group on the terminal alkene).
Ferrier also reported that the final product, compound 5, could be converted into a conjugated ketone (compound 6) by reaction with acetic anhydride (Ac2O) and pyridine, as shown below.
Modifications
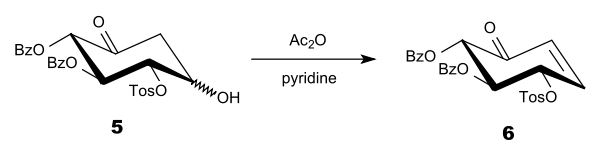
In 1997, Sinaÿ and co-workers reported an alternative route to the synthesis (shown below) that did not involve cleavage of the bond at the anomeric position (the glycosidic bond).[5] In this case, the major product formed had maintained its original configuration at the anomeric position.

Sinaÿ proposed this reaction went through the following transition state:
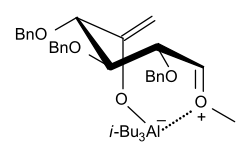
Sinaÿ also discovered that titanium (IV) derivatives such as [TiCl3(OiPr)] worked in the same reaction as a milder version of the Lewis acid, i-Bu3Al,[6] which goes through a similar transition state involving the retention of configuration at the anomeric center.
In 1988, Adam reported a modification of the reaction that used catalytic amounts of palladium (II) salts, which brought about the same conversion of enol ethers into carbosugars in a more environmentally friendly manner.[7]
Applications
The development of the Ferrier carbocyclization has been useful for the synthesis of numerous natural products that contain the carbocycle group. In 1991, Bender and co-workers reported a synthetic route to pure enantiomers of myo-inositol derivatives using this reaction.[8] It has also been applied to the synthesis of aminocyclitols in work done by Barton and co-workers.[9] Finally, Amano et al. used the Ferrier conditions to synthesise complex conjugated cyclohexanones in 1998.[10]
References
- ↑ Ferrier, RJ (1979). "Unsaturated carbohydrates. Part 21. A carbocyclic ring closure of a hex-5-enopyranoside derivative". J. Chem. Soc., Perkin Trans. 1: 1455–1458. doi:10.1039/p19790001455.
- ↑ Blattner, RJ; Ferrier, RJ (1986). "Direct synthesis of 6-oxabicyclo[3.2.1]octane derivatives from deoxyinososes". Carbohydr. Res. 150: 151–162. doi:10.1016/0008-6215(86)80012-X.
- ↑ Ferrier, RJ; Middleton, S (1993). "The conversion of carbohydrate derivatives into functionalized cyclohexanes and cyclopentanes". Chem. Rev. 93 (8): 2779–2831. doi:10.1021/cr00024a008.
- ↑ Marco-Contelles, J; Molina, Maria T.; Anjum, S (2004). "Naturally Occurring Cyclohexane Epoxides: Sources, Biological Activities, and Synthesis†". Chem. Rev. 104 (6): 2857–2900. doi:10.1021/cr980013j. PMID 15186183.
- ↑ Das, SK; Mallet, J-M; Sinaÿ, P (1997). "Novel Carbocyclic Ring Closure of Hex-5-enopyranosides". Angew. Chem. Int. Ed. 36 (5): 493–496. doi:10.1002/anie.199704931.
- ↑ Dalko, PI; Sinaÿ, P (1999). "Recent Advances in the Conversion of Carbohydrate Furanosides and Pyranosides into Carbocycles". Angew. Chem. Int. Ed. 38 (6): 773–777. doi:10.1002/(SICI)1521-3773(19990315)38:6<773::AID-ANIE773>3.0.CO;2-N.
- ↑ Adam, S (1988). "Palladium(II) promoted carbocyclisation of aminodeoxyhex-5-enopyranosides". Tetrahedron Lett. 29 (50): 6589–6592. doi:10.1016/S0040-4039(00)82404-1.
- ↑ Bender, SL; Budhu, RJ (1991). "Biomimetic synthesis of enantiomerically pure D-myo-inositol derivatives". J. Am. Chem. Soc. 113 (26): 9883–9885. doi:10.1021/ja00026a042.
- ↑ Barton, DHR; Camara, J; Dalko, P; Géro, SD; Quiclet-Sire, B; Stütz, P (1989). "Synthesis of biologically active carbocyclic analogs of N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP)". J. Org. Chem. 54 (16): 3764–3766. doi:10.1021/jo00277a002.
- ↑ Amano, S; Ogawa, N; Ohtsuka, M; Ogawa, S; Chida, N (1998). "Total synthesis and absolute configuration of FR65814". Chem. Commun. (12): 1263–1264. doi:10.1039/a802169d.