Enol ether
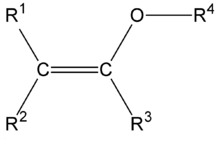
The structure of a typical enol ether group.
An enol ether is an alkene with an alkoxy substituent.[1] The general structure is with R an alkyl or an aryl group. Enol ethers and enamines are so-called activated alkenes or electron rich alkenes because the oxygen atom donates electrons to the double bond by forming a resonance structure with the corresponding oxonium ion. This property makes them reactive substrates in certain organic reactions such as the Diels-Alder reaction. An enol ether can be considered the ether of the corresponding enolate, hence the name. Two simple enol ethers are methyl vinyl ether and 2,3-dihydrofuran.
See also
References
- ↑ Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford University Press. p. 295. ISBN 978-0-19-927029-3.
This article is issued from Wikipedia - version of the 7/21/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.