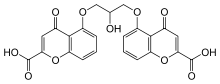
Cromoglicic acid
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category | |
| Routes of administration | topical: oral, nasal spray, inhaled, eye drops |
| ATC code | A07EB01 (WHO) D11AH03 (WHO) R01AC01 (WHO) R03BC01 (WHO) S01GX01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 1% |
| Biological half-life | 1.3 hours |
| Identifiers | |
| |
| CAS Number |
16110-51-3 |
| PubChem (CID) | 2882 |
| IUPHAR/BPS | 7608 |
| DrugBank |
DB01003 |
| ChemSpider |
2779 |
| UNII |
Y0TK0FS77W |
| KEGG |
D07753 |
| ChEBI |
CHEBI:59773 |
| ChEMBL |
CHEMBL74 |
| ECHA InfoCard | 100.036.602 |
| Chemical and physical data | |
| Formula | C23H16O11 |
| Molar mass | 468.367 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Cromoglicic acid (INN) (also referred to as cromolyn (USAN), cromoglycate (former BAN), or cromoglicate) is traditionally described as a mast cell stabilizer, and is commonly marketed as the sodium salt sodium cromoglicate or cromolyn sodium. This drug prevents the release of inflammatory chemicals such as histamine from mast cells.
Because of their convenience (and perceived safety), leukotriene receptor antagonists have largely replaced it as the non-corticosteroid treatment of choice in the treatment of asthma. Cromoglicic acid requires administration four times daily, and does not provide additive benefit in combination with inhaled corticosteroids.[1]
History
Cromolyn sodium was discovered by Roger Altounyan who was himself a lifelong asthma sufferer. It is considered a breakthrough drug in management of asthma, as the patients can be freed from steroids in many cases; however, it is mainly effective as a prophylaxis for allergic and exercise-induced asthma, not as a treatment for acute attacks. Altounyan was investigating certain plants and herbs which have bronchodilating properties. One such plant was khella (Ammi visnaga) which had been used as a muscle relaxant since ancient times in Egypt. Altounyan deliberately inhaled derivatives of the active ingredient khellin to determine if they could block his asthma attacks. After several years of trial he isolated an effective and safe asthma-preventing compound called cromolyn sodium.
Preparations
Cromoglicic acid is available in multiple forms:
- as a nasal spray (Rynacrom(UK), Lomusol(France), Nasalcrom(US) ) to treat allergic rhinitis.
- in a nebulizer solution for aerosol administration to treat asthma.
- as an inhaler (Intal) for preventive management of asthma.[2] The maker of Intal, King Pharmaceuticals, has discontinued manufacturing the inhaled form, cromolyn sodium inhalation aerosol, due to issues involving CFC-free propellant.[3] As stocks are depleted, this inhaler preparation will no longer be available to patients.[4] In the EU it is manufactured without CFCs by Sanofi, although it must be imported from Canada for USA residents.
- as eye drops (Opticrom and Optrex Allergy (UK), Crolom, Cromolyn (Canada)) for allergic conjunctivitis
- in an oral form (Gastrocrom) to treat mastocytosis,[5] dermatographic urticaria and ulcerative colitis. Another oral product, Intercron (sodium cromoglicate in distilled water, from Zambon France), is used for food allergies.
Mechanism of action
"Cromolyn works because it prevents the release of mediators that would normally attract inflammatory cells and because it stabilizes the inflammatory cells. MCT mast cells found in the mucosa are stabilised."[6] Nedocromil is another mast cell stabilizer that also works in controlling asthma. The underlying mechanism of action is not fully understood; for while cromoglicate stabilizes mast cells, this mechanism is probably not why it works in asthma.[7] Pharmaceutical companies have produced 20 related compounds that are equally or more potent at stabilising mast cells and none of them have shown any anti-asthmatic effect.[7] It is more likely that these work by inhibiting the response of sensory C fibers to the irritant capsaicin, inhibiting local axon reflexes involved in asthma, and may inhibit the release of preformed T cell cytokines and mediators involved in asthma. (see review by Garland, 1991)
It is known to somewhat inhibit chloride channels (37% ± 7%)[8] and thus may inhibit the:
- exaggerated neuronal reflexes triggered by stimulation of irritant receptors on sensory nerve endings (e.g. exercise-induced asthma)
- release of preformed cytokines from several type of inflammatory cells (T cells, eosinophils) in allergen-induced asthma
Note: Another chemical (NPPB: 5-nitro-2(3-phenyl) propylamino-benzoic acid) was shown, in the same study, to be a more effective chloride channel blocker.
Finally it may act by inhibiting calcium influx.
Cromoglicate is classified as a chromone.
Cromolyn is also being tested as a drug to treat insulin-induced lipoatrophy.[9][10] Cromolyn is also known to binds S100P protein and disrupts the interaction with RAGE.[11][12]
References
- ↑ Fanta CH (March 2009). "Asthma". New England Journal of Medicine. 360 (10): 1002–14. doi:10.1056/NEJMra0804579. PMID 19264689. Review.
- ↑ Schwartz HJ, Blumenthal M, Brady R, et al. (April 1996). "A comparative study of the clinical efficacy of nedocromil sodium and placebo. How does cromolyn sodium compare as an active control treatment?". Chest. 109 (4): 945–52. doi:10.1378/chest.109.4.945. PMID 8635375.
- ↑ Eric Carter (July 31, 2009). "King Pharmaceuticals: Dear Healthcare Professionals" (PDF). Food and Drug Administration. King Pharmaceuticals. Retrieved May 28, 2012.
- ↑ "Intal Inhaler discontinued - MPR". Empr.com. Retrieved 2012-05-28.
- ↑ Horan RF, Sheffer AL, Austen KF (May 1990). "Cromolyn sodium in the management of systemic mastocytosis". J. Allergy Clin. Immunol. 85 (5): 852–5. doi:10.1016/0091-6749(90)90067-E. PMID 2110198.
- ↑ Werner's Pathophysiology page 224
- 1 2 H. P. Rang et al., Pharmacology, Fifth Edition. (2003) ISBN 0-443-07145-4
- ↑ Heinke, S; Szucs G; Norris A; Droogmans G; Nilius B (August 1995). "Inhibition of volume-activated chloride currents in endothelial cells by chromones". Br J Pharmacol. 115 (8): 1393–8. doi:10.1111/j.1476-5381.1995.tb16629.x. PMC 1908889
 . PMID 8564197.
. PMID 8564197. - ↑ Phua, EJ; Lopez, X; Ramus, J; Goldfine, AB (December 2013). "Cromolyn sodium for insulin-induced lipoatrophy: old drug, new use.". Diabetes Care. 36 (12): e204–5. doi:10.2337/dc13-1123. PMID 24265375.
- ↑ http://www.empr.com/a-surprising-option-for-managing-insulin-induced-lipoatrophy/article/325863/
- ↑ Penumutchu, Srinivasa R.; Chou, Ruey-Hwang; Yu, Chin (2014-08-01). "Structural Insights into Calcium-Bound S100P and the V Domain of the RAGE Complex". PLOS ONE. 9 (8): e103947. doi:10.1371/journal.pone.0103947. ISSN 1932-6203. PMC 4118983
 . PMID 25084534.
. PMID 25084534. - ↑ Penumutchu, Srinivasa R.; Chou, Ruey-Hwang; Yu, Chin (2014-10-17). "Interaction between S100P and the anti-allergy drug cromolyn". Biochemical and Biophysical Research Communications. 454 (3): 404–409. doi:10.1016/j.bbrc.2014.10.048. ISSN 1090-2104. PMID 25450399.