Wenker synthesis
| Wenker synthesis | |
|---|---|
| Named after | Henry Wenker |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | wenker-synthesis |
The Wenker synthesis is an organic reaction converting a beta amino alcohol to an aziridine with the aid of sulfuric acid.[1]
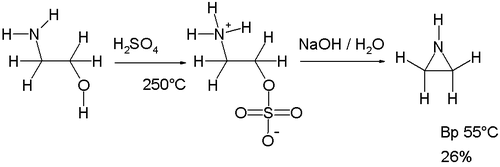
The original Wenker synthesis of aziridine itself takes place in two steps. In the first step ethanolamine is reacted with sulfuric acid at high temperatures (250 °C) to form the sulfate mono-ester. This salt is then reacted with sodium hydroxide in the second step forming aziridine. The base abstracts an amine proton enabling it to displace the sulfate group. A modification of this reaction involving lower reaction temperatures (140–180 °C) and therefore reduced charring increases the yield of the intermediate.[2]
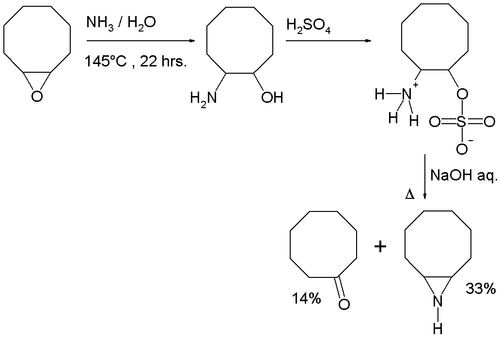
The Wenker synthesis protocol using trans-2-aminocyclooctanol, available from reaction of ammonia with the epoxide of cyclooctene, gives a mixture of cyclooctenimine (the Wenker aziridine product) and cyclooctanone (a competing Hofmann elimination product).[3]
References
- ↑ Henry Wenker (1935). "The Preparation of Ethylene Imine from Monoethanolamine". Journal of the American Chemical Society. 57 (1): 2328–28. doi:10.1021/ja01314a504.
- ↑ A Modification of Wenker's Method of Preparing Ethyleneimine Philip A. Leighton, William A. Perkins, and Melvin L. Renquist J. Am. Chem. Soc.; 1947; 69(6) pp 1540–40. (doi:10.1021/ja01198a512)
- ↑ Chemistry of Ethylenimine. VII. Cycloöctenimine or 9-Azabicyclo[6.1.0]nonane D. V. Kashelikar, Paul E. Fanta J. Am. Chem. Soc.; 1960; 82(18); 4927–30. (doi:10.1021/ja01503a044)