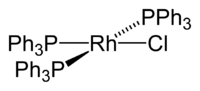
Wilkinson's catalyst
 | |
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC names
(SP-4)chloridotris(triphenylphosphane) rhodium | |
| Other names
Rhodium(I) tris(triphenylphosphine) chloride, Wilkinson's catalyst, Tris(triphenylphosphine)rhodium(I) chloride | |
| Identifiers | |
| 14694-95-2 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.035.207 |
| EC Number | 238-744-5 |
| PubChem | 84599 |
| RTECS number | none |
| |
| Properties | |
| C54H45ClP3Rh | |
| Molar mass | 925.22 g/mol |
| Appearance | red solid |
| Melting point | 245 to 250 °C (473 to 482 °F; 518 to 523 K) |
| insoluble in water | |
| Solubility in other solvents | 20 g/L (CHCl3, CH2Cl2), 2 g/L (benzene, toluene)[1] |
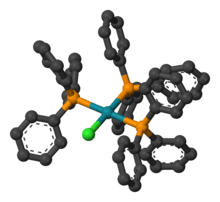
| Structure | |
| square planar | |
| Hazards | |
| Main hazards | none |
| R-phrases | none |
| S-phrases | S22 S24/25 |
| Related compounds | |
| Related compounds |
triphenylphosphine Pd(PPh3)4 IrCl(CO)[P(C6H5)3]2 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Wilkinson's catalyst, is the common name for chloridotris(triphenylphosphane)rhodium(I), an coordination complex of rhodium with the formula RhCl(PPh3)3 (Ph = phenyl). It is a rouge-colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel Laureate, Sir Geoffrey Wilkinson, who first popularized its use.
Historically, Wilkinson's catalyst has been a paradigm in catalytic studies leading to several advances in the field such as the implementation of the some of the first heteronuclear magnetic resonance studies for its structural elucidation in solution (31P),[2] parahydrogen-induced polarization spectroscopy to determine the nature of transient reactive species,[3] or one of the first detailed kinetic investigation by Halpern to elucidate the mechanism.[4] Furthermore, the catalytic and organometallic studies on Wilkinson's catalyst also played a significant role on the subsequent development of cationic Rh- and Ru-based asymmetric hydrogenation transfer catalysts which set the foundations for modern asymmetric catalysis.[5]
Structure and basic properties
According to single crystal X-ray diffraction the compound adopts a slightly distorted square planar structure.[6]
In analyzing the bonding, it is a complex of Rh(I), a d8 transition metal ion. From the perspective of the 18 electron rule, the four ligands each provides two electrons, for a total of 16-electrons. As such the compound is coordinatively unsaturated. Furthermore, in solution the complex undergoes fast dynamic exchange processes both intermolecular (one of the PPh3 is labile and is constantly exchanged between the inner coordination Rh sphere and the solution, although this fast equilibrium is really balanced towards the starting triphosphine complex, K = 10 Exp (-5) M) and intramolecular (fluxionality).[7]
Synthesis
Wilkinson's catalyst is usually obtained by treating rhodium(III) chloride hydrate with an excess of triphenylphosphine in refluxing ethanol.[8][9][1] Triphenylphosphine serves as a two-electron reducing agent that oxidizes itself from oxidation state (III) to (V). In the synthesis, three equivalents of triphenylphosphine become ligands in the product, while the fourth reduces rhodium(III) to rhodium(I).
- RhCl3(H2O)3 + 4 PPh3 → RhCl(PPh3)3 + OPPh3 + 2 HCl + 2 H2O
Catalytic applications
Wilkinson's catalyst is best known for catalyzing the hydrogenation of olefins with molecular hydrogen.[10][11] The mechanism of this reaction involves the initial dissociation of one or two triphenylphosphine ligands to give 14- or 12-electron complexes, respectively, followed by oxidative addition of H2 to the metal. Subsequent π-complexation of alkene, migratory insertion (intramolecular hydride transfer or olefin insertion), and reductive elimination complete the formation of the alkane product, e.g.:
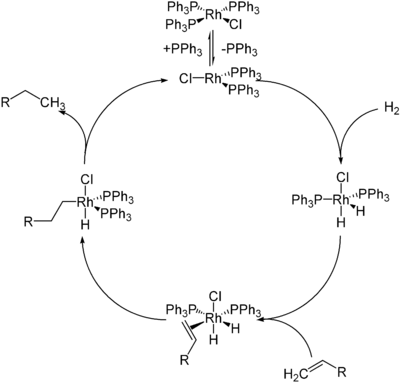
In terms of their rates of hydrogenation, the degree of substitution on the olefin substrate is the key factor, since the rate-limiting step in the mechanism is the insertion into the olefin which is limited by the severe steric hindrance around the metal center. In practice, terminal and disubstituted alkenes are good substrates, but more hindered alkenes are slower to hydrogenate. The hydrogenation of alkynes is troublesome to control since alkynes tend to be reduced to alkanes, via intermediacy of the cis-alkene.[12] Ethylene reacts with Wilkinson's catalyst to give RhCl(C2H4)(PPh3)2, but it is not a substrate for hydrogenation.[9]
Related catalytic processes
Wilkinson's catalyst also catalyzes many other hydrofunctionalization reactions including hydroacylation, hydroboration, and hydrosilylation of alkenes.[12] Hydroborations have been studied with catecholborane and pinacolborane.[13] It is also active for the hydrosilylation of alkenes.[14]
In the presence of strong base and hydrogen, Wilkinson's catalyst forms reactive Rh(I) species with superior catalytic activities on the hydrogenation of internal alkynes and functionalized tri-substituted alkenes.[15]
Organometallic chemistry of Wilkinson's catalyst
RhCl(PPh3)3 reacts with carbon monoxide to give trans-RhCl(CO)(PPh3)2, which is structurally analogous to Vaska's complex (but less reactive). The same complex arises from the decarbonylation of aldehydes:
- RhCl(PPh3)3 + RCHO → RhCl(CO)(PPh3)2 + RH + PPh3
Upon stirring in benzene solution, RhCl(PPh3)3 converts to the poorly soluble red-colored dimer [RhCl(PPh3)2]2. This conversion further demonstrates the lability of the triphenylphosphine ligands.
In the presence of base, H2, and additional triphenylphosphine, Wilkinson's complex converts to HRh(PPh3)4. This 18e complex is also an active hydrogenation catalyst.[16]
See also
References
- 1 2 Osborn, J. A.; Wilkinson, G. (1967). "Tris(triphenylphosphine)halorhodium(I)". Inorg. Synth. 10: 67. doi:10.1002/9780470132418.ch12.
- ↑ Meakin, P.; Jesson, J. P.; Tolman, C. A. (1972-05-01). "Nature of chlorotris(triphenylphosphine)rhodium in solution and its reaction with hydrogen". Journal of the American Chemical Society. 94 (9): 3240–3242. doi:10.1021/ja00764a061. ISSN 0002-7863.
- ↑ Duckett, Simon B.; Newell, Connie L.; Eisenberg, Richard. "Observation of New Intermediates in Hydrogenation Catalyzed by Wilkinson's Catalyst, RhCl(PPh3)3, Using Parahydrogen-Induced Polarization". Journal of the American Chemical Society. 116 (23): 10548–10556. doi:10.1021/ja00102a023.
- ↑ Halpern, Jack (1981-01-01). "Mechanistic aspects of homogeneous catalytic hydrogenation and related processes". Inorganica Chimica Acta. 50: 11–19. doi:10.1016/S0020-1693(00)83716-0.
- ↑ Hartwig, John F. (2010). Organotransition metal chemistry- From bonding to Catalysis. University Science Books. ISBN 978-1-891389-53-5.
- ↑ Bennett, Michael J.; Donaldson, Peter B. "Crystal and molecular structure of the orange and red allotropes of chlorotris(triphenylphosphine)rhodium(I)" Inorganic Chemistry 1977, volume 16, pp. 655-60. doi:10.1021/ic50169a033
- ↑ Moberg, Christina (2011-10-24). "Stereomutation in Trigonal-Bipyramidal Systems: A Unified Picture". Angewandte Chemie International Edition. 50 (44): 10290–10292. doi:10.1002/anie.201103375. ISSN 1521-3773.
- ↑ Bennett, M. A.; Longstaff, P. A. (1965). "Complexes of Rhodium(I) with Triphenylphosphine.". Chem. Ind. (London). 1965: 846.
- 1 2 Osborn, J. A.; Jardine, F. H.; Young, J. F.; Wilkinson, G. (1966). "The Preparation and Properties of Tris(triphenylphosphine)halogenorhodium(I) and Some Reactions Thereof Including Catalytic Homogeneous Hydrogenation of Olefins and Acetylenes and Their Derivatives". J. Chem. Soc. A. 1966: 1711–1732. doi:10.1039/J19660001711.
- ↑ Birch, A. J.; Williamson, D. H. (1976). "Homogeneous Hydrogenation Catalysts in Organic Synthesis". Org. React. 24: 1.
- ↑ James, B. R. (1973). Homogeneous Hydrogenation. New York, NY: John Wiley & Sons.
- 1 2 Kevin Burgess, Wilfred A. van der Donk, Chul-Ho Jun, Young Jun Park, "Chlorotris(triphenylphosphine)-rhodium(I)" Encyclopedia of Reagents for Organic Synthesis 2005 John Wiley & Sons. doi:10.1002/047084289X.rc162s.pub2
- ↑ Evans, D. A.; Fu, G. C.; Hoveyda, A. H. (1988). "Rhodium(I)-catalyzed hydroboration of olefins. The documentation of regio- and stereochemical control in cyclic and acyclic systems". J. Am. Chem. Soc. 110 (20): 6917–6918. doi:10.1021/ja00228a068.
- ↑ Ojima, I.; Kogure, T. (1972). "Selective reduction of α,β-unsaturated terpene carbonyl compounds using hydrosilane-rhodium(I) complex combinations". Tetrahedron Lett. 13 (49): 5035–5038. doi:10.1016/S0040-4039(01)85162-5.
- ↑ Perea Buceta, Jesus E.; Fernández, Israel; Heikkinen, Sami; Axenov, Kirill; King, Alistair W. T.; Niemi, Teemu; Nieger, Martin; Leskelä, Markku; Repo, Timo (2015-11-23) [2015]. "Diverting Hydrogenations with Wilkinson's Catalyst towards Highly Reactive Rhodium(I) Species". Angewandte Chemie International Edition,. Wiley. 54 (48): 14321–14325. doi:10.1002/anie.201506216. ISSN 1521-3773.
- ↑ Eduardo Peña-Cabrera "Hydridotetrakis(triphenylphosphine)rhodium" Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons. doi:10.1002/047084289X.rh030m