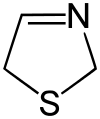
Thiazoline
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC names
2,3-Dihydrothiazole 2,5-Dihydrothiazole 4,5-Dihydrothiazole | |
| Other names
2,3-Dihydro-1,3-thiazole or 4-thiazoline 2,5-Dihydro-1,3-thiazole or 3-thiazoline 4,5-Dihydro-1,3-thiazole or 2-thiazoline | |
| Identifiers | |
| 504-79-0 (2,3) 24576-55-4 (2,5) 504-79-0 (4,5) | |
| 3D model (Jmol) | (2,3): Interactive image (2,5): Interactive image (4,5): Interactive image |
| PubChem | 151424 (2,3) 120269 (4,5) |
| |
| Properties | |
| C3H5NS | |
| Molar mass | 87.14 g·mol−1 |
| Appearance | Colorless liquids |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Thiazolines (or dihydrothiazoles) are a group of isomeric heterocyclic compound containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more common and some are bioactive. For example, in a common post-translational modification, cysteine residues are converted into thiazolines.[1]
Synthesis
Thiazolines were first prepared by dialkylation of thioamides.[2] More commonly, they are prepared from derivatives of 2-aminoethanethiol (cysteamine).
Related compounds
Three related classes of C3NS heterocycles are well studied, 1,3-thiazoles (parent: C3H3NS), 1,3-thiazolines (parent: C3H5NS), and 1,3-thiazolidines (parent: C3H7NS). The naming is analogous to the C3N2 heterocycles, imidazoles, imidazolines, and imidazolidines.
Substituted thiazolines
Many molecules contain thiazoline rings, one example being luciferin, the light-emitting molecule in fireflies. The amino acid cysteine is produced industrially from substituted thiazole.[3]
Thiazolines found in nature
In a recent study, thiazolines were identified in nature through an analysis of sesame seed oil. The toasted sesame seed oil was extracted using a Solvent-Assisted Flavor Evaporation technique. The extract was analyzed by GC and GC-MS and a total of 87 components were identified. Amongst these components, 2-ethyl-4-methyl-3-thiazoline and 2-isopropyl-4-methyl-3-thiazoline were identified and confirmed as being present in a natural product for the first time.[4]
See also
References
- ↑ Walsh, Christopher T.; Nolan, Elizabeth M. (2008). "Morphing peptide backbones into heterocycles". Proceedings of the National Academy of Sciences USA. 105: 5655–5656. doi:10.1073/pnas.0802300105.
- ↑ Willstätter, Richard; Wirth, Theodor (1909). "Über Thioformamid". Chem. Ber. 42: 1908–1922. doi:10.1002/cber.19090420267.
- ↑ Gaumont, Annie-Claude; Gulea, Mihaela; Levillain, Jocelyne (2009). "Overview of the Chemistry of 2-Thiazolines". Chem. Rev. 109: 1371–1401. doi:10.1021/cr800189z.
- ↑ Agyemang, D.; Bardsley, K.; Brown, S.; Kraut, K.; Psota-Kelty, L.; Trinnaman, L. (2011). "Identification of 2-Ethyl-4-Methyl-3-Thiazoline and 2-Isopropyl-4-Methyl-3-Thiazoline for the First Time in Nature by the Comprehensive Analysis of Sesame Seed Oil". J. Food Sci. 76: C385–C391. doi:10.1111/j.1750-3841.2011.02071.x.