Tetrarhodium dodecacarbonyl
12.png) | |
 | |
| Names | |
|---|---|
| IUPAC names
tri-μ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl-
1κ2C,2κ2C,3κ2C,4κ3C-[Td-(13)-Δ4-closo]- tetrarhodium(6 Rh—Rh) | |
| Other names
rhodium(0) carbonyl; rhodium carbonyl; rhodium dodecacarbonyl | |
| Identifiers | |
| 19584-30-6 | |
| ECHA InfoCard | 100.039.232 |
| Properties | |
| Rh4(CO)12 | |
| Molar mass | 747.743 g/mol |
| Appearance | Red crystals |
| Solubility | Chlorocarbons, toluene, tetrahydrofuran |
| Related compounds | |
| Related compounds |
Rhodium(III) chloride, Rh6(CO)16, Rh2(CO)4Cl2 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
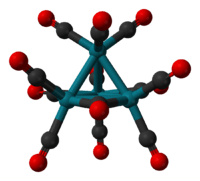
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest stable binary rhodium carbonyl. It is used as a catalyst in organic synthesis.
Structure, synthesis, reactions
The structure of Rh4(CO)12 is described by a tetrahedral array of four Rh atoms with nine terminal CO ligands and three bridging CO ligands. The structure can be expressed as Rh4(CO)9(µ-CO)3.
It is prepared by treatment of an aqueous solution of rhodium trichloride with activated copper metal under an atmosphere of CO.[1]
- 4 RhCl3(H2O)3 + 8 Cu + 22 CO → Rh4(CO)12 + 2 CO2 + 8 Cu(CO)Cl + 4 HCl + 10 H2O
Alternatively, the compound can be prepared by treatment of a methanolic solution of RhCl3(H2O)3 with CO to afford H[RhCl2(CO)2], followed by carbonylation in the presence of sodium citrate.[2]
The cluster undergoes thermal substitution with phosphorus ligands:
- Rh4(CO)12-n + n L → Rh4(CO)12-nLn + n CO
Related metal carbonyls
Because of their relevance to hydroformylation catalysis, the metal carbonyls has been systematically studied to a high degree. The instability of Rh2(CO)8 has been a source of curiosity. The analogous binary carbonyl of cobalt, Co2(CO)8, is well known. Solutions of Rh4(CO)12 under high pressures of CO convert to the dirhodium compound:[3]
- Rh4(CO)12 + 4 CO → 2 Rh2(CO)8
Unlike Co2(CO)8, the main isomer of Rh2(CO)8 features only terminal CO ligands. The relative instability of Rh2(CO)8 is analogous to the tendency of Ru(CO)5 to convert to Ru3(CO)12.
References
- ↑ S. Martinengo; G. Giordano; P. Chini; G. W. Parshall; E. R Wonchoba (1990). Robert J. Angelico, ed. "Tri-µ-carbonyl-nonacarbonyltetrarhodium". Inorganic Syntheses. 28: 242–245. doi:10.1002/9780470132593.ch62.
- ↑ Serp, P.; Kalck, P.; Feurer, R.; Morancho, R. (1998). Marcetta. Y. Darensbourg, ed. "Tri-µ-carbonyl-nonacarbonyltetrarhodium". Inorganic Syntheses. 32: 284–287. doi:10.1002/9780470132630.ch45.
- ↑ Brown, D. T.; Eguchi, T.; Heaton, B. T.; Iggo, J. A.; Whyman, R. (1991). "High-pressure spectroscopic studies of reactions of the clusters [Rh4(CO)12–x{P(OPh)3}x] (x= 1–4) with carbon monoxide or syngas". Journal of the Chemical Society, Dalton Transactions: 677–683. doi:10.1039/DT9910000677.
General reading
- King, R. B., "Rhodium: Organometallic Chemistry" Encyclopedia of Inorganic Chemistry 1994, 7, 3494.