Tetraphenylethylene
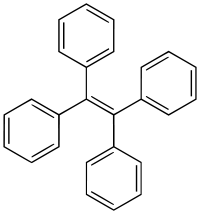 | |
| Names | |
|---|---|
| IUPAC name
1,2,2-triphenylvinylbenzene | |
| Other names
tetraphenylethylene, tetraphenylethene, 1,1,2,2-tetraphenylethylene, 1,1,2,2-tetraphenylethene, tetraphenyl ethylene | |
| Identifiers | |
| 632-51-9 | |
| 3D model (Jmol) | Interactive image |
| 789087 | |
| ChemSpider | 62645 |
| ECHA InfoCard | 100.010.164 |
| PubChem | 69437 |
| |
| |
| Properties | |
| C26H20 | |
| Molar mass | 332.45 g·mol−1 |
| Appearance | white to light yellow to light beige crystalline powder |
| Density | 1.088 g/cm3 |
| Melting point | 224 to 225 °C (435 to 437 °F; 497 to 498 K)[1] |
| Boiling point | 424 °C (795 °F; 697 K)[2] |
| Hazards | |
| Flash point | 206.2 °C (403.2 °F; 479.3 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetraphenylethylene is a chemical compound that can be used in construction and in the manufacture of medical equipment, packaging, and electrical appliances.
Synthesis
Tetraphenylethylene can be synthesized from diphenyldichloromethane.[3]
References
- ↑ Banerjee, Moloy; Susanna J. Emond; Sergey V. Lindeman; Rajendra Rathore (2007). "Practical Synthesis of Unsymmetrical Tetraarylethylenes and Their Application for the Preparation of [Triphenylethylene−Spacer−Triphenylethylene] Triads". The Journal of Organic Chemistry. 72 (21): 8054–8061. doi:10.1021/jo701474y. ISSN 0022-3263.
- ↑ Lewis, Irwin C.; T. Edstrom (1963). "Thermal Reactivity of Polynuclear Aromatic Hydrocarbons". The Journal of Organic Chemistry. 28 (8): 2050–2057. doi:10.1021/jo01043a025. ISSN 0022-3263.
- ↑ Inaba, S (1982). "Metallic nickel as a reagent for the coupling of aromatic and benzylic halides". Tetrahedron Letters. 23 (41): 4215–4216. doi:10.1016/S0040-4039(00)88707-9. ISSN 0040-4039.
This article is issued from Wikipedia - version of the 3/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.