Structural scheduling of synthetic cannabinoids
To combat the illicit synthetic cannabinoid industry many states have created a system to control these cannabinoids through their structure as opposed to their identity (or name). In this way new analogs are already controlled before they are even created.
For example, Kentucky controls Naphthoylindoles: Any compound containing a 3-(1-naphthoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl,or2-(4-morpholinyl)ethylgroup, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent.[1]
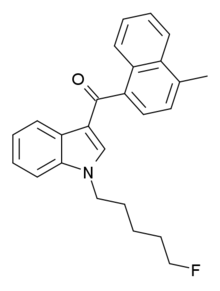
This causes a substance such as MAM-2201 to be controlled as a Schedule 1 non-narcotic, even though "MAM-2201" does not appear in the law. Notice the five carbon chain with the fluorine atom attached to the Nitrogen atom. This is covered under "substitution at the nitrogen atom of the indole ring by an..... haloakyl". The methane group on the napthyl group is covered under "whether or not substituted in the napthyl ring to any extent". It is in this way MAM-2201 can be controlled without being named in the statute.
Kentucky:
Beginning in 2011 the Kentucky Legislature added section 45 to KRS 218A.010 in order to control the variety of synthetic cannabinoid analogs that were emerging as "legal" substitutes for marijuana. This law now includes naphthoylindoles, phenylacetylindoles, benzoylindoles, cyclohexylphenols, naphthylmethylindoles, naphthoylpyrroes, naphthylmethylindenes each with specific substitutions on specific atoms of the molecule. Examples are given in each instance to help identify the molecules described.
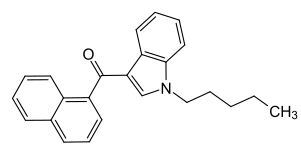
Naphthoylindoles: Any compound containing a 3-(1-naphthoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl,or2-(4-morpholinyl)ethylgroup, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent.[1]
One example given is JWH-018, one of the earliest synthetic cannabinoids identified. Notice the indole ring has an alkyl substitution on the nitrogen atom.
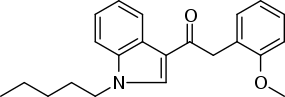
Phenylacetylindoles: Any compound containing a 3-phenylacetylindole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl,1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.[2]

One example given is JWH-250. There is an alkyl substitution on the nitrogen atom of the indole ring as well as a methoxy group attached to the phenyl ring.
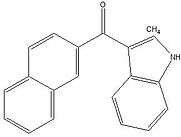
Benzoylindoles: Any compound containing a 3-(benzoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.[3]
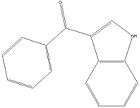
One example is RCS-4. Note the alkyl group substitution on the Nitrogen atom of the indole. It is further substituted in the phenyl ring with a methanone group.

Cyclohexylphenols: Any compound containing a 2-(3-hydroxycyclohexyl)phenol structure with substitution at the 5-position of the phenolic ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not substituted in the cyclohexyl ring to any extent.[4]
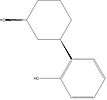
One example is CP 47,497. Notice the methyloctan-2-yl group substituted onto the phenol group of the molecule.

Naphthylmethylindoles: Any compound containing a 1H-indol-3-yl-(1-naphthyl)methane structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent.[5]

One example is JWH-175. Notice the pentyl group substituted onto the nitrogen atom of the indole ring.

Naphthoylpyrroles: Any compound containing a 3-(1-naphthoyl)pyrrole structure with substitution at the nitrogen atom of the pyrrole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent.[6]

One example is JWH-030. Notice the pentyl group on the nitrogen atom of the pyrrole ring of the molecule.

Naphthylmethylindenes: Any compound containing a 1-(1-naphthylmethyl)indene structure with substitution at the 3-position of the indene ring by an alkyl, haloalkyl, alkenyl, cloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent.[7]
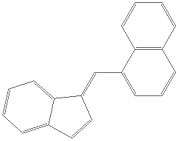
One example is JWH-176. Notice the 5 membered pentyl group on the indene ring.

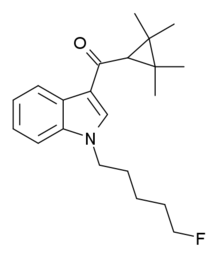
Tetramethylcyclopropanoylindoles: Any compound containing a 3-(1-tetramethylcyclopropoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not further substituted in the tetramethylcyclopropyl ring to any extent.[8]
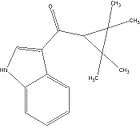
One example is XLR-11. Notice the 5 membered alkyl group ending with a fluorine atom substituted onto the nitrogen atom of the indole group.
Adamantoylindoles: Any compound containing a 3-(1-adamantoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl,or2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the adamantyl ring system to any extent.[9]

One example is AB-001. Notice the 5-membered alkyl group on the nitrogen atom of the indole ring.

Indole-3-carboxylate esters: Any compound containing a 1H-indole-3-carboxylate ester structure with the ester oxygen bearing a napthyl, quinolinyl, isoquinolinyl, or adamantyl group and substitution at the one (1) position of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, or benzyl groups to any extent.[10]
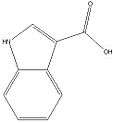
One example is PB-22. Notice the quinolinyl group attached to the oxygen atom and the 5 carbon chain (pentyl) group on the Nitrogen atom.

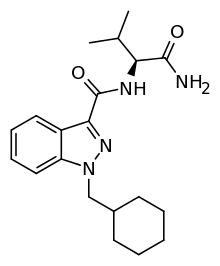
Indazole-3-carboxamides: Any compound containing a 1H-indazole-3-carboxamide structure with substitution at the nitrogen of the carboxamide by a naphthyl, quinolinyl, isoquinolinyl, adamantyl, or 1-amino-1-oxoalkan-2-yl group and substitution at the one (1) position of the indazole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indazole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, 1-amino-oxoalkan-2-yl, or benzyl groups to any extent.[11]

One example is AB-CHMINACA. Notice the indole group has a cyclohexylmethyl (a type of cycloalkylmethyl) group attached at the nitrogen atom. Also there is an 1-amino-1-oxoalkan-2-yl group (1-amino-3-methyl-1-oxobutan-2-yl in this instance) substituted on the nitrogen atom of the carboxamide group.