Separatory funnel

A separatory funnel, also known as separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (partition) the components of a mixture into two immiscible solvent phases of different densities.[1] Typically, one of the phases will be aqueous, and the other a non-polar lipophilic organic solvent such as ether, MTBE, dichloromethane, chloroform, or ethyl acetate. All of these solvents form a clear delineation between the two liquids.[2] The two layers formed are usually known as the organic and aqueous phases.[3] Most organic solvents float on top of an aqueous phase, though important exceptions are most halogenated solvents.[4] The organic solvent used for the extraction must not react with the substance to be extracted or with water. It should also have a low boiling point so it can be easily removed from the product.[5]
Description

A separating funnel takes the shape of a cone with a hemispherical end. It has a stopper at the top and stopcock (tap), at the bottom. Separating funnels used in laboratories are typically made from borosilicate glass and their stopcocks are made from glass or PTFE. Typical sizes are between 30 mL and 3 L. In industrial chemistry they can be much bigger and for much larger volumes centrifuges are used. The sloping sides are designed to facilitate the identification of the layers. The stopcock-controlled outlet is designed to drain the liquid out of the funnel. On top of the funnel there is a standard taper joint which fits with a ground glass or Teflon stopper.[6]
To use a separatory funnel, the two phases and the mixture to be separated in solution are added through the top with the stopcock at the bottom closed. The funnel is then closed and shaken gently by inverting the funnel multiple times; if the two solutions are mixed together too vigorously emulsions will form. The funnel is then inverted and the tap carefully opened to release excess vapor pressure. The separating funnel is set aside to allow for the complete separation of the phases. The top and the bottom tap are then opened and the lower phase is released by gravitation. The top must be opened while releasing the lower phase to allow pressure equalization between the inside of the funnel and the atmosphere. When the bottom layer has been removed, the stopcock is closed and the upper layer is poured out through the top into another container.
Before using the separatory funnel, make sure it is placed safely in a ring stand. Also place an Erlenmeyer flask below the separatory funnel to ensure that any drops which may leak out of the funnel are caught in the flask. Finally, it is of vital importance to make sure that the stopcock is tightly closed.[7]
Theory
The separatory funnel runs on the concept of "like dissolves like". This means that if a compound is polar,[8] then it can only be dissolved in a polar solvent. Following this logic, a non-polar compound can only be dissolved in a non-polar solvent.[9] In a separatory funnel, there are two phases, the aqueous layer is polar, and the organic layer is non-polar. Because these two layers share a surface, any polar substances that were initially in the solution will be pulled into the polar/aqueous layer, and any non-polar substances will be pulled into the non-polar/organic layer. The denser of the two layers is then removed via the stopcock.[10] The vast majority of the time water is used for the polar layer, so the position of the organic layer depends on its relative density to that of water.[11] There should be a distinct phase boundary between the two layers, however if an emulsion (vide infra) forms, it may take time for the layers to completely separate. Once the bottom layer is removed and the desired layer is isolated, a recrystallization is used to isolate the target compound. Because a recrystallization commonly follows a separation and it is often the organic layer that is isolated, an organic layer with a low boiling point is preferable to reduce the evaporation time.[12]
Emulsions
Emulsions can be formed while liquids are being mixed in the separatory funnel. This can occur when small droplets are suspended in an aqueous solution. If an emulsion is formed, try to slowly swirl the solution in the separatory funnel. If the emulsion is not eliminated, then try adding a small amount of saturated aqueous sodium chloride solution.[13] The main drawback to the use of a separatory funnel is that oftentimes, it takes significant time for the emulsion to dissipate. Research is being done on alternative, more efficient techniques, mostly utilizing stir bars to decrease or even eliminate the chance of emulsification, thus decreasing the amount of waiting time.[14]
Safety concerns
The largest risk when using a separatory funnel is that of pressure build-up. Pressure accumulates during mixing if gas evolving reactions occur. This problem can be easily handled by simply opening the stopper at the top of the funnel routinely while mixing. This should be done with the top of the funnel pointed away from the body.[15] When shaking, hold the stopper in place or it can become dislodged causing the liquids to spill. To account for this, simply hold the stopper in place with one hand.[16]
Gallery
-
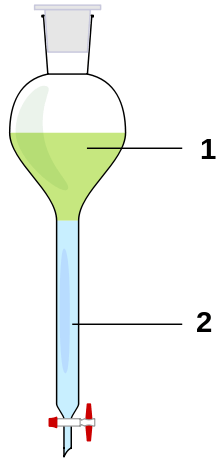
1. Upper phase
2. Lower phase -

The ether layer with a dissolved yellow compound is on top and an aqueous layer is at the bottom
See also
- Dropping funnels are similar in shape and design, and may be used as separatory funnels. They have standard taper ground glass joints at the stem.
- Partition coefficient is a measure of the distribution of an analyte between the two phases in a separation.
References
- ↑ http://www.tutorvista.com/content/chemistry/chemistry-i/elements-compounds/separating-funnel-use.php
- ↑ http://chem.allegheny.edu/chem231/sep%20funnel%20primer.pdf
- ↑ http://chem.allegheny.edu/chem231/sep%20funnel%20primer.pdf
- ↑ http://www.chem.ucla.edu/%7Ebacher/General/30BL/tips/Sepfunnel.html
- ↑ http://www.wellesley.edu/Chemistry/chem211lab/Orgo_Lab_Manual/Appendix/Techniques/Extraction/extraction.html
- ↑ http://orgchem.colorado.edu/equipment/glassware/sepfun.html
- ↑ http://faculty.ycp.edu/~ttao/Home/Extraction.html
- ↑ http://dwb.unl.edu/Teacher/NSF/C06/C06Links/www.uis.edu/7Etrammell/organic/introduction/polarity.htm
- ↑ http://www.kentchemistry.com/links/bonding/LikeDissolveslike.htm
- ↑ Padìas, Anne B. (2011). Making the Connections2: A How-To Guide for Organic Chemistry Lab Techniques.Plymouth, Michigan: Hayden-McNeil Publishing, p. 129.
- ↑ http://sitesmedia.s3.amazonaws.com/chem/files/2012/01/sep-funnel-primer.pdf
- ↑ https://www.erowid.org/archive/rhodium/chemistry/equipment/recrystallization.html
- ↑ http://www.uwplatt.edu/chemep/chem/chemscape/labdocs/catofp/mixpour/sepfunl/sepfunl.htm
- ↑ "A novel and fast extraction technique as an alternative to conventional separatory funnels". Fresenius' Journal of Analytical Chemistry. 345: 411–414. doi:10.1007/BF00325616.
- ↑ Fessenden, J., Joan S. Fessenden, Patty Feist. Organic Laboratory Techniques, 3rd Edition, 2001. Pacific Grove, California: Brooks Cole Publishing, p. 59.
- ↑ http://www.chem.ucla.edu/~bacher/General/30BL/tips/Sepfunnel.html