Selenium in biology
Although it is toxic in large doses, selenium is an essential micronutrient for animals. In plants, it sometimes occurs in amounts toxic as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase.[1] Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).
Se-containing biomolecules
Selenium is an essential micronutrient in mammals, but is also recognized as toxic in excess. Selenium exerts its biological functions through selenoproteins, which contain the amino acid selenocysteine. Twenty-five selenoproteins are encoded in the human genome.[2]
Glutathione peroxidase
The glutathione peroxidase family of enzymes (abbreviated GSH-Px) catalyze reduction of hydrogen peroxide and organic hydroperoxides:
- 2H + H2O2 → GSSG + 2 H2O
The two H atoms are donated by thiols in a process that begins with oxidation of a selenol side chain in GSH-Px. The organoselenium compound ebselen is a drug used to supplement the action of GSH-Px. It functions as a catalyst for the destruction of hydrogen peroxide.[3]
A related selenium-containing enzyme in some plants and in animals (thioredoxin reductase) generates reduced thioredoxin, a dithiol that serves as an electron source for peroxidases and also the important reducing enzyme ribonucleotide reductase that makes DNA precursors from RNA precursors.[4]
Deiodinases
Selenium also plays a role in the functioning of the thyroid gland. It participates as a cofactor for the three thyroid hormone deiodinases. These enzymes activate and then deactivate various thyroid hormones and their metabolites.[5] It may inhibit Hashimotos's disease, an auto-immune disease in which the body's own thyroid cells are attacked by the immune system. A reduction of 21% on TPO antibodies was reported with the dietary intake of 0.2 mg of selenium.[6]
Formate dehydrogenase
Some microorganisms ulitize selenium in formate dehydrogenase. Formate is produced in large amounts in the hepatic (liver cells) mitochondria of embryonic cells and in cancer cells by the folate cycle.[7]
Formate is reversibly oxidized by the enzyme formate dehydrogenase:[8]
- HCO2− → CO2 + H+ + 2 e−
Nutritional sources of selenium
Dietary selenium comes from nuts, cereals, meat, mushrooms, fish, and eggs. Brazil nuts are the richest ordinary dietary source and could cause selenium toxicity if consumed regularly – though the actual concentration of selenium, as with any plant-based food sources such as another selenium-accumulating "paradise nut" Lecythis, belonging to the same family Lecythidaceae, is soil-dependent and may vary significantly by geographic location. In descending order of concentration, high levels are also found in kidney, tuna, crab, and lobster.[9][10]
The human body's content of selenium is believed to be in the 13–20 milligram range.[11]
Indicator plants
Certain species of plants are considered indicators of high selenium content of the soil, since they require high levels of selenium to thrive. The main selenium indicator plants are Astragalus species (including some locoweeds), prince's plume (Stanleya sp.), woody asters (Xylorhiza sp.), and false goldenweed (Oonopsis sp.)[12]
Medical use of synthetic selenium compounds
The substance loosely called selenium sulfide (with the approximate formula SeS2) is the active ingredient in some anti-dandruff shampoos.[13] The selenium compound kills the scalp fungus Malassezia, which causes shedding of dry skin fragments. The ingredient is also used in body lotions to treat Tinea versicolor due to infection by a different species of Malassezia fungus.[14]
Detection in biological fluids
Selenium may be measured in blood, plasma, serum or urine to monitor excessive environmental or occupational exposure, confirm a diagnosis of poisoning in hospitalized victims or to assist in a forensic investigation in a case of fatal overdosage. Some analytical techniques are capable of distinguishing organic from inorganic forms of the element. Both organic and inorganic forms of selenium are largely converted to monosaccharide conjugates (selenosugars) in the body prior to being eliminated in the urine. Cancer patients receiving daily oral doses of selenothionine may achieve very high plasma and urine selenium concentrations.[15]
Toxicity
Although selenium is an essential trace element, it is toxic if taken in excess. Exceeding the Tolerable Upper Intake Level of 400 micrograms per day can lead to selenosis.[16] This 400 microgram (µg) Tolerable Upper Intake Level is based primarily on a 1986 study of five Chinese patients who exhibited overt signs of selenosis and a follow up study on the same five people in 1992.[17] The 1992 study actually found the maximum safe dietary Se intake to be approximately 800 micrograms per day (15 micrograms per kilogram body weight), but suggested 400 micrograms per day to not only avoid toxicity, but also to avoid creating an imbalance of nutrients in the diet and to account for data from other countries.[18] In China, people who ingested corn grown in extremely selenium-rich stony coal (carbonaceous shale) have suffered from selenium toxicity. This coal was shown to have selenium content as high as 9.1%, the highest concentration in coal ever recorded in literature.[19]
Symptoms of selenosis include a garlic odor on the breath, gastrointestinal disorders, hair loss, sloughing of nails, fatigue, irritability, and neurological damage. Extreme cases of selenosis can result in cirrhosis of the liver, pulmonary edema, and death.[20] Elemental selenium and most metallic selenides have relatively low toxicities because of their low bioavailability. By contrast, selenates and selenites are very toxic, having an oxidant mode of action similar to that of arsenic trioxide. The chronic toxic dose of selenite for humans is about 2400 to 3000 micrograms of selenium per day for a long time.[21] Hydrogen selenide is an extremely toxic, corrosive gas.[22] Selenium also occurs in organic compounds, such as dimethyl selenide, selenomethionine, selenocysteine and methylselenocysteine, all of which have high bioavailability and are toxic in large doses.
Selenium poisoning of water systems may result whenever new agricultural runoff courses through normally dry, undeveloped lands. This process leaches natural soluble selenium compounds (such as selenates) into the water, which may then be concentrated in new "wetlands" as the water evaporates. High selenium levels produced in this fashion have been found to have caused certain congenital disorders in wetland birds.[23]
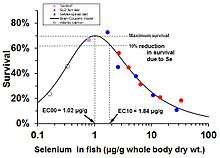
In fish and other wildlife, low levels of selenium cause deficiency while high levels cause toxicity. For example, in salmon, the optimal concentration of selenium in the fish tissue (whole body) is about 1 microgram selenium per gram of tissue (dry weight). At levels much below that concentration, young salmon die from selenium deficiency;[24] much above that level they die from toxic excess.[25]
Deficiency
Selenium deficiency is rare in healthy, well-nourished individuals. It can occur in patients with severely compromised intestinal function, those undergoing total parenteral nutrition, and[26] in those of advanced age (over 90). Also, people dependent on food grown from selenium-deficient soil are at risk. Although New Zealand has low levels of selenium in its soil, adverse health effects have not been detected.[27]
Selenium deficiency as defined by low (<60% of normal) selenoenzyme activity levels in brain and endocrine tissues only occurs when a low selenium status is linked with an additional stress, such as high exposures to mercury[28] or as a result of increased oxidant stress due to vitamin E deficiency.[29]
Selenium interacts other nutrients, such as iodide and vitamin E. The interaction is observed in the etiology of many deficiency diseases in animals and pure selenium deficiency is rare. The effect of selenium deficiency on health remains uncertain, particularly in relation to Kashin-Beck disease.[30]
Human health
Cancer
No good evidence indicates selenium supplementation helps prevent cancer.[31]
HIV/AIDS
Some research has indicated a geographical link between regions of selenium-deficient soils and peak incidences of HIV/AIDS infection. For example, much of sub-Saharan Africa is low in selenium. However, Senegal is not, and also has a significantly lower level of AIDS infection than the rest of the continent. AIDS appears to involve a slow and progressive decline in levels of selenium in the body. Whether this decline in selenium levels is a direct result of the replication of HIV[32] or related more generally to the overall malabsorption of nutrients by AIDS patients remains debated.
Observational studies have found an association between decreased selenium levels and poorer outcomes in patients with HIV, though these studies were mostly done prior to the currently effective treatments with highly active antiretroviral therapy, or HAART. Currently there is inadequate evidence to recommend routine selenium supplementation for HIV patients, and further research is recommended.[33]
Mortality
Selenium supplementation has no effect on overall mortality.[34]
Tuberculosis
As with other types of supplementation, there is no good evidence selenium supplementation helps in the treatment of tuberculosis.[35]
Diabetes
A well-controlled study showed selenium levels are positively correlated with the risk of having type 2 diabetes. Because high serum selenium levels are positively associated with the prevalence of diabetes, and because selenium deficiency is rare, supplementation is not recommended in well-nourished populations, such as the U.S.[36] More recent studies, however, have indicated selenium may help inhibit the development of type 2 diabetes in men, though the mechanism for the possible preventative effect is not known.[37]
Human reproductive system
Abnormal levels of dietary selenium can have an adverse effect on sperm quality, with a consequent lowering of fertility.[38]
Evolution in biology and biosynthetic considerations
Selenium is incorporated into several prokaryotic selenoprotein families in bacteria, archaea, and eukaryotes as selenocysteine,[39] where selenoprotein peroxiredoxins protect bacterial and eukaryotic cells against oxidative damage. Selenoprotein families of GSH-Px and the deiodinases of eukaryotic cells seem to have a bacterial phylogenetic origin. The selenocysteine-containing form occurs in species as diverse as green algae, diatoms, sea urchin, fish and chicken. Selenium enzymes are involved in utilization of the small reducing molecules glutathione and thioredoxin.
Trace elements involved in GSH-Px and superoxide dismutase enzymes activities, i.e. selenium, vanadium, magnesium, copper, and zinc, may have been lacking in some terrestrial mineral-deficient areas.[39] Marine organisms retained and sometimes expanded their seleno-proteomes, whereas the seleno-proteomes of some terrestrial organisms were reduced or completely lost. These findings suggest that aquatic life supports selenium utilization, whereas terrestrial habitats lead to reduced use of this trace element.[40][41] Marine fishes and vertebrate thyroid glands have the highest concentration of selenium and iodine. From about 500 Mya, freshwater and terrestrial plants slowly optimized the production of "new" endogenous antioxidants such as ascorbic acid (Vitamin C), polyphenols (including flavonoids), tocopherols, etc. A few of these appeared more recently, in the last 50–200 million years, in fruits and flowers of angiosperm plants. In fact, the angiosperms (the dominant type of plant today) and most of their antioxidant pigments evolved during the late Jurassic period.
About 200 Mya, new selenoproteins were developed as mammalian GSH-Px enzymes.[42][43][44][45]
See also
References
- ↑ S. J. Lippard, J. M. Berg "Principles of Bioinorganic Chemistry" University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ↑ Kurokawa, Suguru; Berry, Marla J. (2013). Astrid Sigel, Helmut Sigel and Roland K. O. Sigel, ed. Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. 13. Springer. pp. 499–534 Selenium. Role of the Essential Metalloid in Health. doi:10.1007/978-94-007-7500-8_16.
- ↑ Bhabak Krishna P., Mugesh Govindasamy; Mugesh (2010). "Functional Mimics of Glutathione Peroxidase: Bioinspired Synthetic Antioxidants". Acc. Chem. Res. 43 (11): 1408–1419. doi:10.1021/ar100059g. PMID 20690615.
- ↑ Stadtman TC (1996). "Selenocysteine". Annual Review of Biochemistry. 65: 83–100. doi:10.1146/annurev.bi.65.070196.000503. PMID 8811175.
- ↑ "Selenium". Linus Pauling Institute at Oregon State University. Retrieved 2009-01-05.
- ↑ Mazokopakis, EE; Papadakis, JA; Papadomanolaki, MG; Batistakis, AG; Giannakopoulos, TG; Protopapadakis, EE; Ganotakis, ES (2007). "Effects of 12 months treatment with L-selenomethionine on serum anti-TPO Levels in Patients with Hashimoto's thyroiditis". Thyroid. 17 (7): 609–12. doi:10.1089/thy.2007.0040. PMID 17696828.
- ↑ H Frederik Nijhout, et al, In silico experimentation with a model of hepatic mitochondrial folate metabolism, Theoretical Biology and Medical Modeling, 2006, 3:40, link http://www.tbiomed.com/content/3/1/40/abstract).
- ↑ Reda T., Plugge C. M., Abram N. J., Hirst J.; Plugge; Abram; Hirst (2008). "Reversible interconversion of carbon dioxide and formate by an electroactive enzyme". PNAS. 105 (31): 10654–10658. doi:10.1073/pnas.0801290105. PMC 2491486
 . PMID 18667702.
. PMID 18667702. - ↑ Barclay, Margaret N. I.; Allan MacPherson; James Dixon (1995). "Selenium content of a range of UK food". Journal of food composition and analysis. 8 (4): 307–318. doi:10.1006/jfca.1995.1025.
- ↑ A list of selenium-rich foods can be found on The Office of Dietary Supplements Selenium Fact Sheet.
- ↑ The most popular web reference for this is .
- ↑ Zane Davis, T. (2008). "Selenium in Plants" (PDF). p. 8. Retrieved 2008-12-05.
- ↑ "Selenium(IV)_sulfide". Pharmacy Codes. Retrieved 2009-01-06.
- ↑ "Selenium sulfide". DermNet NZ. Retrieved 2009-01-06.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1416–1420.
- ↑ "Dietary Supplement Fact Sheet: Selenium". National Institutes of Health; Office of Dietary Supplements. Retrieved 2009-01-05.
- ↑ a report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Leves of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. (August 15, 2000). Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Institute of Medicine. pp. 314–315. ISBN 0-309-06949-1.
- ↑ Yang, G.; Zhou, R. (1994). "Further Observations on the Human Maximum Safe Dietary Selenium Intake in a Seleniferous Area of China". Journal of trace elements and electrolytes in health and disease. 8 (3–4): 159–165. PMID 7599506.
- ↑ Yang, Guang-Qi; Xia, Yi-Ming (1995). "Studies on Human Dietary Requirements and Safe Range of Dietary Intakes of Selenium in China and Their Application in the Prevention of Related Endemic Diseases". Biomedical and Environmental Sciences. 8 (3): 187–201. PMID 8561918.
- ↑ "Public Health Statement: Health Effects" (PDF). Agency for Toxic Substances and Disease Registry. Retrieved 2009-01-05.
- ↑ Wilber, C. G. (1980). "Toxicology of selenium". Clinical Toxicology. 17 (2): 171–230. doi:10.3109/15563658008985076. PMID 6998645.
- ↑ Olson, O.E. (1986). "Selenium Toxicity in Animals with Emphasis on Man". International Journal of Toxicology. 5: 45. doi:10.3109/10915818609140736.
- ↑ Ohlendorf, H. M. (2003). "Ecotoxicology of selenium". Handbook of ecotoxicology. Boca Raton: Lewis Publishers. pp. 466–491. ISBN 978-1-56670-546-2.
- ↑ Poston, H. A.; Combs, G. F.; Leibovitz, L. (1976). "Vitamin E and selenium interrelations in the diet of Atlantic salmon (Salmo salar): gross, histological and biochemical signs". Journal of Nutrition. 106: 892–904.
- ↑ Hamilton, Steven J.; K. J. Buhl, N. L. Faerber, R. H. Wiedmeyer, and F. A. Bullard (1990). "Toxicity of organic selenium in the diet to chinook salmon". Environ. Toxicol. Chem. 9 (3): 347–358. doi:10.1002/etc.5620090310.
- ↑ Ravaglia; Forti, P; Maioli, F; Bastagli, L; Facchini, A; Mariani, E; Savarino, L; Sassi, S; et al. (1 February 2000). "Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged >=90 y1". American Journal of Clinical Nutrition. 71 (2): 590–598. PMID 10648276.
- ↑ MedSafe Editorial Team. "Selenium". Prescriber Update Articles. New Zealand Medicines and Medical Devices Safety Authority. Retrieved 2009-07-13.
- ↑ Ralston, N.V.C.; Raymond, L.J. (2010). "Dietary selenium's protective effects against methylmercury toxicity". Toxicology. 278: 112–123. doi:10.1016/j.tox.2010.06.004. PMID 20561558.
- ↑ Mann, Jim; Truswell, A. Stewart (2002). Essentials of Human Nutrition (2nd ed.). Oxford University Press. ISBN 978-0-19-262756-8.
- ↑ Moreno-Reyes, Rodrigo; Mathieu, Jean; Vanderpas, Marleen; Begaux, Françoise; Suetens, Carl; Rivera, Maria T.; Nève, Jean; Perlmutter, Noémi; V (2003). "Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy". American Journal of Clinical Nutrition. 78 (1): 137–144. PMID 12816783.
- ↑ Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MP, Horneber M (2011). "Selenium for preventing cancer". Cochrane Database Syst Rev (5): CD005195. doi:10.1002/14651858.CD005195.pub2. PMC 3692366
 . PMID 21563143.
. PMID 21563143. - ↑ Patrick L (1999). "Nutrients and HIV: part one – beta carotene and selenium" (PDF). Alternative Medicine Review. 4 (6): 403–13. PMID 10608913.
- ↑ Stone CA, Kawai K, Kupka R, Fawzi WW (November 2010). "Role of selenium in HIV infection". Nutr. Rev. 68 (11): 671–81. doi:10.1111/j.1753-4887.2010.00337.x. PMC 3066516
 . PMID 20961297.
. PMID 20961297. - ↑ Bjelakovic, G; Nikolova, D; Gluud, LL; Simonetti, RG; Gluud, C (2012). Bjelakovic, Goran, ed. "Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases". Cochrane database of systematic reviews (Online). 3: CD007176. doi:10.1002/14651858.CD007176.pub2. PMID 22419320.
- ↑ Sinclair D, Abba K, Grobler L, Sudarsanam TD (2011). "Nutritional supplements for people being treated for active tuberculosis". Cochrane Database Syst Rev (Systematic review) (11): CD006086. doi:10.1002/14651858.CD006086.pub3. PMID 22071828.
- ↑ Bleys J, Navas-Acien A, Guallar E (2007). "Serum selenium and diabetes in U.S. adults". Diabetes Care. 30 (4): 829–34. doi:10.2337/dc06-1726. PMID 17392543.
- ↑ Selenium Protects Men Against Diabetes, Study Suggests
- ↑ Ahsan U, Kamran Z, Raza I, et al. (April 2014). "Role of selenium in male reproduction - a review". Anim. Reprod. Sci. (Review). 146 (1-2): 55–62. doi:10.1016/j.anireprosci.2014.01.009. PMID 24613013.
- 1 2 Gladyshev VN, Hatfield DL (1999). "Selenocysteine-containing proteins in mammals". Journal of Biomedical Science. 6 (3): 151–60. doi:10.1007/BF02255899. PMID 10343164.
- ↑ Lobanov AV, Fomenko DE, Zhang Y, Sengupta A, Hatfield DL, Gladyshev VN (2007). "Evolutionary dynamics of eukaryotic selenoproteomes: large selenoproteomes may associate with aquatic life and small with terrestrial life". Genome Biology. 8 (9): R198. doi:10.1186/gb-2007-8-9-r198. PMC 2375036
 . PMID 17880704.
. PMID 17880704. - ↑ Penglase, Sam; Hamre, Kristin; Ellingsen, Ståle. "The selenium content of SEPP1 versus selenium requirements in vertebrates". PeerJ. 3. doi:10.7717/peerj.1244. PMC 4699779
 . PMID 26734501.
. PMID 26734501. - ↑ Castellano S, Novoselov SV, Kryukov GV, et al. (2004). "Reconsidering the evolution of eukaryotic selenoproteins: a novel nonmammalian family with scattered phylogenetic distribution". EMBO Reports. 5 (1): 71–7. doi:10.1038/sj.embor.7400036. PMC 1298953
 . PMID 14710190.
. PMID 14710190. - ↑ Kryukov GV, Gladyshev VN (2004). "The prokaryotic selenoproteome". EMBO Reports. 5 (5): 538–43. doi:10.1038/sj.embor.7400126. PMC 1299047
 . PMID 15105824.
. PMID 15105824. - ↑ Wilting R, Schorling S, Persson BC, Böck A (1997). "Selenoprotein synthesis in archaea: identification of an mRNA element of Methanococcus jannaschii probably directing selenocysteine insertion". Journal of Molecular Biology. 266 (4): 637–41. doi:10.1006/jmbi.1996.0812. PMID 9102456.
- ↑ Zhang Y, Fomenko DE, Gladyshev VN (2005). "The microbial selenoproteome of the Sargasso Sea". Genome Biology. 6 (4): R37. doi:10.1186/gb-2005-6-4-r37. PMC 1088965
 . PMID 15833124.
. PMID 15833124.
External links
| Wikimedia Commons has media related to Selenium. |
| Look up selenium in Wiktionary, the free dictionary. |
- WebElements.com – Selenium
- National Institutes of Health page on Selenium
- Assay
- ATSDR – Toxicological Profile: Selenium
- Peter van der Krogt elements site
| Periodic table (Large cells) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
|
| |||||||||||||||||||||||||||||||||