Sarett oxidation
| Sarett oxidation | |
|---|---|
| Named after | Lewis Hastings Sarett |
| Reaction type | Organic redox reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000547 |
The Sarett oxidation is an organic reaction that oxidizes primary and secondary alcohols to aldehydes and ketones, respectively, using chromium trioxide and pyridine. Unlike the similar Jones oxidation, the Sarett oxidation will not further oxidize primary alcohols to their carboxylic acid form, neither will it affect carbon-carbon double bonds.[1] Use of the original Sarett oxidation has become largely antiquated however, in favor of other modified oxidation techniques. The unadulterated reaction is still occasionally used in teaching settings and in small scale laboratory research.[2]

History
First appearance
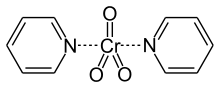
The reaction is named after the American chemist Lewis Hastings Sarett (1917–1999). The first description of its use appears in a 1953 article[3] co-authored by Sarett that relates to the synthesis of adrenal steroids. The paper proposes the use of the pyridine chromium complex CrO3-2C5H5N to oxidize primary and secondary alcohols. The complex would later become known as the "Sarett Reagent".
Modifications and improvements
Although the Sarett reagent gives good yields of ketones, its conversion of primary alcohols is less efficient. Furthermore, the isolation of products from the reaction solution can be difficult.[4] These limitations lead to the 1968 introduction of the Collins oxidation, which improved overall yields and separation for primary alcohols.[4] The so-called "Collins reagent" is actually identical to the Sarett reagent (CrO3-2C5H5N). The Collins oxidation varies from the Sarett oxidation only in that it uses methylene chloride as an improved solvent for the Sarett reagent, instead of the original pyridine.[4] The initially proposed methods of executing the Collins and Sarett oxidations were still not ideal however, as the Sarett reagent's hygroscopic, and pyrophoric properties make it difficult to prepare.[5] This issues lead to an improvement of the Collins oxidation protocol in 1970 known as the Ratcliffe variant, which is now the most commonly used oxidation technique that uses the Sarett reagent.[5][6]
Preparation of the Sarett reagent
Techniques
-oxid.jpg)
The Sarett reagent was originally prepared in 1953 by addition of chromic anhydride to pyridine[3] First, reagent grade pyridine must be cooled to 15–20 °C. Then, a small initial portion of the chromic anhydride is carefully stirred into to the pyridine. Eventually, the brick-red anhydride will transform into the orange-yellow Sarett reagent in an exothermic reaction. At this point the remaining anhydride may be gradually stirred into the solution. This must be done very slowly so that the mixture never rises above 30 °C. The final product is a slurry of excess pyridine and the precipitated Sarett reagent, which can take up to an hour to prepare (per 100 g yield). The Sarett oxidation would then be carried out immediately in this mixture.[3]
Safety

The specific methods of the reagent's preparation are critical, as improper technique can cause the explosion of the materials.[6] Some technical improvements to the original methodology have reduced the risks associated with preparation. One such recent improvement reduced the likelihood of explosion by using chromic anhydride granules that would immediately sink below the surface of the cooled pyridine upon addition.[2] It should also be mentioned that chromium trioxide is a corrosive carcinogen and therefore must be handled with extreme care.[7]
Collins technique
The original Collins oxidation calls for the Sarett reagent to be removed from the excess pyridine and dissolved in the less basic methylene chloride.[4][6] While the new solvent improves the overall yield of the reaction, it also requires the dangerous transfer of the pyrophoric reagent. The 1970 Ratcliffe variation reduced the risk of explosion by calling for the Sarett reagent to be made in situ. This was achieved by creating the Sarett reagent according to the original protocol using a stirred mixture of pyridine and methylene chloride.[5]
Specific applications
The Sarett oxidation efficiently oxidizes primary alcohols to aldehydes without further oxidizing them to carboxylic acids.[6] This key difference from the Jones oxidation is due to the fact that the Jones oxidation occurs in the presence of water, which adds to the alcohol following oxidation to an aldehyde.[6][8] The Sarett and Collins oxidations occur in the absence of water.[6] The Sarett oxidation also proceeds under basic conditions, which allows for the use of acid sensitive substrates, such as those containing certain protecting groups. This is dissimilar to other common acidic oxidation reactions such as the Baeyer-Villiger oxidation, which would remove or alter such groups. Additionally, the Sarett reagent is relatively inert towards double bonds and thioether groups.[3] These groups cannot effectively interact with the chromium of the Sarett reagent, as compared to the chromium in oxidizing complexes used prior to 1953.[3]
See also
References
- ↑ Margareta Avram (1983). "Chimie organica" p. 472. "Editura Academiei Republicii Socialiste România"
- 1 2 Nagappam, Arumugam (January 2003). "A facile synthesis of 2-aroylindoles by the oxidation of 2-arylmethylindoles using Sarett reagent". Synthetic Communications. 33 (12): 2313–2320. doi:10.1081/SCC-120021513.
- 1 2 3 4 5 Poos, G. I.; Arth, G. E.; Beyler, R. E.; Sarett, L. H. (1953). "Approaches to the Total Synthesis of Adrenal Steroids.1V. 4b-Methyl-7- ethylenedioxy-1,2,3,4,4aα,4b,5,6,7,8,10,10a β-dodecahydrophenanthrene-4 β-ol-1-one and Related Tricyclic Derivatives". Journal of the American Chemical Society. 75 (2): 422. doi:10.1021/ja01098a049.
- 1 2 3 4 Collins, J.C.; W.W. Hess; F.J. Frank (1968). "Dipyridine-chromium(VI) oxide oxidation of alcohols in dichloromethane". Tetrahedron Letters. 9 (30): 3363–3366. doi:10.1016/s0040-4039(00)89494-0.
- 1 2 3 Ratcliffe, R; Rodehorst, R. (1970). "Improved procedure for oxidations with the chromium trioxide-pyridine complex". Journal of Organic Chemistry. 35 (11): 4000–4002. doi:10.1021/jo00836a108.
- 1 2 3 4 5 6 Tojo, Gabriel; Fernández, Marcos (2006). Oxidation of alcohols to aldehydes and ketones a guide to current common practice. New York, NY: Springer. ISBN 978-0-387-23607-0.
- ↑ "Chromium Trioxide (MSDS)". J. T. Baker. Retrieved 2007-09-13.
- ↑ "Jones Oxidation". organic-chemistry.org.