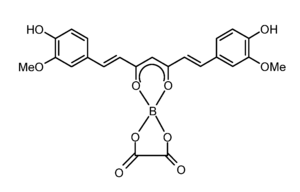
Rubrocurcumin
 | |
| Names | |
|---|---|
| Other names
Rubrocurcumin | |
| Identifiers | |
| 12098-66-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 24751750 Non-charged form 24751749 Charged form |
| |
| |
| Properties | |
| C23H19BO10 | |
| Molar mass | 466.19 g/mol |
| Appearance | red solid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Rubrocurcumin is a red colored dye that is formed by the reaction of curcumin and borates.
Synthesis
The reaction of curcumin with borates in presence of oxalic acid produces rubrocurcumin.[1]
Characteristics
Rubrocurcumin produces a red colored solution.
Rubrocurcumin is a neutrally charged composition, while rosocyanine is build from ions. In rubrocurcumin, one molecule curcumin is replaced with oxalate compared to rosocyanine.
Complexes with boron such as rubrocurcumin are called 1,3,2-dioxaborines.[1]
Literature
- Spicer, G. S.; Strickland, J. D. H. (1952). "Compounds of Curcumin and Boric Acid. Part II. The Structure of Rubrocurcumin". Journal of the Chemical Society. London. 1952 (article 907): 4650–4653. doi:10.1039/JR9520004650.
References
- 1 2 Rohde, D. (2002). "Darstellung und Eigenschaftsuntersuchungen an 1,3,2-Dioxaborinen mit variablen Coliganden am Boratom (Dissertation)". University Halle.
This article is issued from Wikipedia - version of the 11/29/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.