Rigosertib
 | |
| Names | |
|---|---|
| IUPAC name
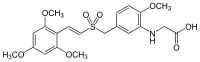
2‐[(2‐Methoxy‐5‐{[(E)‐2‐(2,4,6‐trimethoxyphenyl)ethenesulfonyl]methyl}phenyl)amino]acetic acid | |
| Other names
ON-01910 | |
| Identifiers | |
| 1225497-78-8 | |
| 3D model (Jmol) | [1]: Interactive image |
| ChEMBL | ChEMBL1241855 ChEMBL2013119 |
| ChemSpider | 5293927 |
| 7833 | |
| PubChem | 6918736 |
| Properties | |
| C21H25NO8S | |
| Molar mass | 451.49 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rigosertib (ON-01910 sodium salt, with Estybon as trade name) is a synthetic benzyl styryl sulfone that is in phase III clinical trials as an anti-cancer agent.
Its geometrical isomer (Z)-ON 01910·Na has less cytotoxicity on cancer cells.
Mechanism
Rigosertib is a small molecule inhibitor, which simultaneously inhibits PI3K and PLK signaling pathways.The over-expression of these two pathways may lead occurrence and development of many kinds of tumors.[3] Thus rigosertib performs potential antineoplastic activity in multiple tumor types.
Rigosertib can convert the gene express profilings, cause mitotic cell-cycle G2 arrest of tumor cells, leading their apoptosis. And what it causes in normal cells is a reversible cell arrest at the G1 and G2 stage without apoptosis. Rigosertib shows little liver damage or neurotoxicity in mouse xenograft models.
Rigosertib is an non-ATP-competitive inhibitor. It inhibits PLK1 by competing at substrate-binding sites with an IC50 of 9 nM.[4]
References
- ↑ "physical and chemical data on chemispider website".
- ↑ "physical and chemical data on chemispider website".
- ↑ Nuthalapati S (Sep 2012). "Preclinical pharmacokinetic and pharmacodynamic evaluation of novel anticancer agents, ON01910.Na (Rigosertib, Estybon™) and ON013105, for brain tumor chemotherapy.". Pharm Res. 29 (9). doi:10.1007/s11095-012-0780-y. PMID 22678771.
- ↑ "Rigosertib activity data in vitro and in vivo". selleckchemicals. 20 Aug 2014.