Potassium pyrosulfate
 | |
| Names | |
|---|---|
| IUPAC name
dipotassium (sulfonatooxy)sulfonate | |
| Other names
Potassium pyrosulphate; potassium disulfate | |
| Identifiers | |
| 7790-62-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 56432 |
| ECHA InfoCard | 100.029.288 |
| PubChem | 62681 |
| |
| |
| Properties | |
| K2O7S2 | |
| Molar mass | 254.31 g·mol−1 |
| Density | 2.28 g/cm3 |
| Melting point | 325 °C (617 °F; 598 K) |
| soluble | |
| Hazards | |
| R-phrases | R36 R38 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
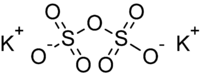
Potassium pyrosulfate, or potassium disulfate, is an inorganic compound with the chemical formula K2S2O7.
Production
Potassium pyrosulfate is obtained by the thermal decomposition of other salts, most directly from potassium bisulfate:[1]
- 2 KHSO4 → K2S2O7 + H2O
Temperatures above 600°C further decompose potassium pyrosulfate to potassium sulfate and sulfur trioxide however:[2]
- K2S2O7 → K2SO4 + SO3
Other salts, such as potassium trisulfate,[3] can also decompose into potassium pyrosulfate.
Chemical Structure
Potassium pyrosulfate contains the pyrosulfate anion which has a dichromate like structure. The geometry can be visualized as a tetrahedron with two corners sharing the SO4 anion's configuration and a centrally bridged oxygen atom.[4] A semi-structural formula for the pyrosulfate anion is O3SOSO32−. The oxidation state of sulfur in this compound is +6.
Uses
Potassium pyrosulfate is used in analytical chemistry; samples are fused with potassium pyrosulfate, (or a mixture of potassium pyrosulfate and potassium fluoride) to ensure complete dissolution prior to a quantitative analysis.[5][6]
The compound is also used as a catalyst in conjunction with vanadium(V) oxide in the industrial production of sulfur trioxide.[7]
See also
References
- ↑ Washington Wiley, Harvey (1895). Principles and Practice of Agricultural Analysis: Fertilizers. Easton, PA.: Chemical Publishing Co. p. 218. Retrieved 31 December 2015.
- ↑ Iredelle Dillard Hinds, John (1908). Inorganic Chemistry: With the Elements of Physical and Theoretical Chemistry. New York: John Wiley & Sons. p. 547. Retrieved 31 December 2015.
- ↑ Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry Vol. 2, 2nd Ed. Newyork: Academic Press. p. 1716. ISBN 9780323161299.
- ↑ Ståhl, K.; Balic-Zunic, T.; da Silva, F.; Eriksen, K. M.; Berg, R. W.; Fehrmann, R. (2005). "The crystal structure determination and refinements of K2S2O7, KNaS2O7 and Na2S2O7 from X-ray powder and single crystal diffraction data". Journal of Solid State Chemistry. 178 (5): 1697–1704. Bibcode:2005JSSCh.178.1697S. doi:10.1016/j.jssc.2005.03.022.
- ↑ Trostbl, L. J.; Wynne, D. J. (1940). "Determination of quartz (free silica) in refractory clays". Journal of the American Ceramic Society. 23 (1): 18–22. doi:10.1111/j.1151-2916.1940.tb14187.x.
- ↑ Sill, C. W. (1980). "Determination of gross alpha, plutonium, neptunium, and/or uranium by gross alpha counting on barium sulphate". Analytical Chemistry. 52 (9): 1452–1459. doi:10.1021/ac50059a018.
- ↑ Burkhardt, Donald (1965). "Sulfur trioxide production, US3362786A". Google Patents. Retrieved 31 December 2015.