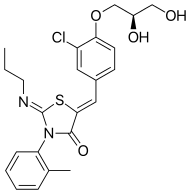
Ponesimod
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | 2 main metabolites |
| Biological half-life | 31–34 hrs[1] |
| Excretion | Feces (57–80%, 26% unchanged), urine (10–18%)[2] |
| Identifiers | |
| |
| Synonyms | ACT-128800 |
| CAS Number | 854107-55-4 |
| PubChem (CID) | 11363176 |
| ChemSpider | 9538103 |
| ChEMBL | CHEMBL1096146 |
| Chemical and physical data | |
| Formula | C23H25ClN2O4S |
| Molar mass | 460.974 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Ponesimod (INN, codenamed ACT-128800) is an experimental drug for the treatment of multiple sclerosis (MS) and psoriasis. It is being developed by Actelion.
Clinical trials
In a 2009–2011 Phase II clinical trial including 464 MS patients, ponesimod treatment resulted in fewer new active brain lesions than placebo, measured during the course of 24 weeks.[3][4]
In a 2010–2012 Phase II clinical trial including 326 patients with psoriasis, 46 or 48% of patients (depending on dosage) had a reduction of at least 75% Psoriasis Area and Severity Index (PASI) score compared to placebo in 16 weeks.[3][5]
Adverse effects
Common adverse effects in studies were temporary bradycardia (slow heartbeat), usually at the beginning of the treatment, dyspnoea (breathing difficulties), and increased liver enzymes (without symptoms). No significant increase of infections was observed under ponesimod therapy.[3] QT prolongation is detectable but was considered to be too low to be of clinical importance in a study.[6]
Mechanism of action
Like fingolimod, which is already approved for the treatment of MS, ponesimod blocks the sphingosine-1-phosphate receptor. This mechanism prevents lymphocytes (a type of white blood cells) from leaving lymph nodes.[3] Ponesimod is selective for subtype 1 of this receptor, S1P1.[7]
See also
References
- ↑ "Multiple-dose tolerability, pharmacokinetics, and pharmacodynamics of ponesimod, an S1P1 receptor modulator: Favorable impact of dose up-titration". The Journal of Clinical Pharmacology. 54: 179–88. Feb 2014. doi:10.1002/jcph.244. PMID 24408162.
- ↑ "Mass balance, pharmacokinetics and metabolism of the selective S1P1 receptor modulator ponesimod in humans". Xenobiotica. 45: 139–49. Feb 2015. doi:10.3109/00498254.2014.955832. PMID 25188442.
- 1 2 3 4 H. Spreitzer (29 September 2014). "Neue Wirkstoffe – Ponesimod". Österreichische Apothekerzeitung (in German) (20/2014): 42.
- ↑ "Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial". Journal of Neurology, Neurosurgery. 85: 1198–208. Nov 2014. doi:10.1136/jnnp-2013-307282. PMC 4215282
 . PMID 24659797.
. PMID 24659797. - ↑ "Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial". The Lancet. 384: 2036–45. Dec 2014. doi:10.1016/S0140-6736(14)60803-5. PMID 25127208.
- ↑ "Effect of Ponesimod, a selective S1P1 Receptor Modulator, on the QT Interval in Healthy Subjects". Basic. 116: 429–37. May 2015. doi:10.1111/bcpt.12336. PMID 25287214.
- ↑ "Ponesimod". Actelion. Retrieved 31 October 2014.