Poly(beta-D-mannuronate) lyase
In enzymology, a poly(beta-D-mannuronate) lyase (EC 4.2.2.3) is an enzyme that catalyzes the chemical reaction
- Eliminative cleavage of polysaccharides containing beta-D-mannuronate residues to give oligosaccharides with 4-deoxy-alpha-L-erythro-hex-4-enopyranuronosyl groups at their ends
This enzyme belongs to the family of lyases, specifically those carbon-oxygen lyases acting on polysaccharides. The systematic name of this enzyme class is poly(beta-D-1,4-mannuronide) lyase. Other names in common use include alginate lyase I, alginate lyase, alginase I, alginase II, and alginase. This enzyme participates in fructose and mannose metabolism.
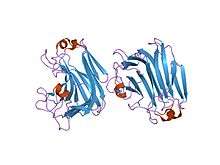
Structural studies
As of late 2007, 3 structures have been solved for this class of enzymes, with PDB accession codes 1HV6, 1J1T, and 2CWS.
References
- Davidson IW, Lawson CJ, Sutherland IW (1977). "An alginate lysate from Azotobacter vinelandii phage". J. Gen. Microbiol. 98 (1): 223–9. doi:10.1099/00221287-98-1-223. PMID 13144.
- Nakada HI, Sweeny PC (1967). "Alginic acid degradation by eliminases from abalone hepatopancreas". J. Biol. Chem. 242 (5): 845–51. PMID 6020438.
- Preiss J; Ashwell G (1962). "Alginic acid metabolism in bacteria. I. Enzymatic formation of unsaturated oligosaccharides and 4-deoxy-L-erythro-5-hexoseulose uronic acid". J. Biol. Chem. 237: 309–316.
|
|---|
|
| Activity | |
|---|
|
| Regulation | |
|---|
|
| Classification | |
|---|
|
| Types | |
|---|