Physiology of decompression
The physiology of decompression involves a complex interaction of gas solubility, partial pressures and concentration gradients, diffusion, bulk transport and bubble mechanics in living tissues.[1] Gas is breathed at ambient pressure, and some of this gas dissolves into the blood and other fluids. Inert gas continues to be taken up until the gas dissolved in the tissues is in a state of equilibrium with the gas in the lungs, (see: "Saturation diving"), or the ambient pressure is reduced until the inert gases dissolved in the tissues are at a higher concentration than the equilibrium state, and start diffusing out again.[2]
The absorption of gases in liquids depends on the solubility of the specific gas in the specific liquid, the concentration of gas, customarily measured by partial pressure, and temperature.[2] In the study of decompression theory the behaviour of gases dissolved in the tissues is investigated and modeled for variations of pressure over time.[3] Once dissolved, distribution of the dissolved gas may be by diffusion, where there is no bulk flow of the solvent, or by perfusion where the solvent (blood) is circulated around the diver's body, where gas can diffuse to local regions of lower concentration. Given sufficient time at a specific partial pressure in the breathing gas, the concentration in the tissues will stabilise, or saturate, at a rate depending on the solubility, diffusion rate and perfusion. If the concentration of the inert gas in the breathing gas is reduced below that of any of the tissues, there will be a tendency for gas to return from the tissues to the breathing gas. This is known as outgassing, and occurs during decompression, when the reduction in ambient pressure or a change of breathing gas reduces the partial pressure of the inert gas in the lungs.[2]
The combined concentrations of gases in any given tissue will depend on the history of pressure and gas composition. Under equilibrium conditions, the total concentration of dissolved gases will be less than the ambient pressure, as oxygen is metabolised in the tissues, and the carbon dioxide produced is much more soluble. However, during a reduction in ambient pressure, the rate of pressure reduction may exceed the rate at which gas can be eliminated by diffusion and perfusion, and if the concentration gets too high, it may reach a stage where bubble formation can occur in the supersaturated tissues. When the pressure of gases in a bubble exceed the combined external pressures of ambient pressure and the surface tension from the bubble - liquid interface, the bubbles will grow, and this growth can cause damage to tissues. Symptoms caused by this damage are known as Decompression sickness.[2]
The actual rates of diffusion and perfusion, and the solubility of gases in specific tissues are not generally known, and vary considerably. However mathematical models have been proposed which approximate the real situation to a greater or lesser extent, and these models are used to predict whether symptomatic bubble formation is likely to occur for a given pressure exposure profile.[3]
Solubility
Solubility is the property of a gas, liquid or solid substance (the solute) to be held homogeneously dispersed as molecules or ions in a liquid or solid medium (the solvent). In decompression theory the solubility of gases in liquids is of primary importance, as it is the formation of bubbles from these gases that causes decompression sickness.[4][5][6]
Solubility of gases in liquids is influenced by three main factors:
- The nature of the solvent liquid and the solute [7]
- Temperature (gases are less soluble in water but may be more soluble in organic solvents, at higher temperatures.)[8][9]
- Pressure (solubility of a gas in a liquid is proportional to the partial pressure of the gas on the liquid – Henry's Law)[10]
The presence of other solutes in the solvent can also influence solubility.[11]
Body tissues include aqueous and lipid components in varying ratios, and the solubility of the gases involved in decompression in these tissues will vary depending on their composition.[12]
| Gas | Molecular weight | Lipid/water solubility ratio |
|---|---|---|
| Hydrogen | 2 | 3.1 |
| Helium | 4 | 1.7 |
| Neon | 20 | 2.07 |
| Nitrogen | 28 | 5.2 |
Diffusion
Diffusion is the movement of molecules or ions in a medium when there is no gross mass flow of the medium, and can occur in gases, liquids or solids, or any combination.[13] Diffusion is driven by the kinetic energy of the diffusing molecules – it is faster in gases and slower in solids when compared with liquids due to the variation in distance between collisions, and diffusion is faster when the temperature is higher as the average energy of the molecules is greater. Diffusion is also faster in smaller, lighter molecules of which helium is the extreme example. Diffusivity of helium is 2.65 times faster than nitrogen.[14]
The partial pressure gradient, also known as the concentration gradient, can be used as a model for the driving mechanism of diffusion. The partial pressure gradient is the variation of partial pressure (or more accurately, the concentration) of the solute (dissolved gas) from one point to another in the solvent. The solute molecules will randomly collide with the other molecules present, and tend over time to spread out until the distribution is statistically uniform. This has the effect that molecules will diffuse from regions of higher concentration (partial pressure) to regions of lower concentration, and the rate of diffusion is proportional to the rate of change of the concentration. Tissues in which an inert gas is more soluble will eventually develop a higher dissolved gas content than tissues where the gas is less soluble.[15]
Inert gas uptake (Ingassing)
.svg.png)
In this context, inert gas refers to a gas which is not metabolically active. Atmospheric nitrogen (N2) is the most common example, and helium (He) is the other inert gas commonly used in breathing mixtures for divers.[16]
Atmospheric nitrogen has a partial pressure of approximately 0.78 bar at sea level. Air in the alveoli of the lungs is diluted by saturated water vapour (H2O) and carbon dioxide (CO2), a metabolic product given off by the blood, and contains less oxygen (O2) than atmospheric air as some of it is taken up by the blood for metabolic use. The resulting partial pressure of nitrogen is about 0,758 bar.[17]
At atmospheric pressure the body tissues are therefore normally saturated with nitrogen at 0.758 bar (569 mmHg). At increased ambient pressures due to depth or habitat pressurisation, a diver's lungs are filled with breathing gas at the increased pressure, and the partial pressures of the constituent gases will be increased proportionately.[3]
- For example: At 10 meters sea water (msw) the partial pressure of nitrogen in air will be 1.58 bar.[3]
The inert gases from the breathing gas in the lungs diffuse into blood in the alveolar capillaries ("move down the pressure gradient") and are distributed around the body by the systemic circulation in the process known as perfusion.[3]
Perfusion
Perfusion is the mass flow of blood through the tissues. Dissolved materials are transported in the blood much faster than they would be distributed by diffusion alone (order of minutes compared to hours).[18]
The dissolved gas in the alveolar blood is transported to the body tissues by the blood circulation. There it diffuses through the cell membranes and into the tissues, where it may eventually reach equilibrium. The greater the blood supply to a tissue, the faster it will reach equilibrium with gas at the new partial pressure.[3][18]
Saturation and supersaturation
If the supply of gas to a solvent is unlimited, the gas will diffuse into the solvent until there is so much dissolved that equilibrium is reached and the amount diffusing back out is equal to the amount diffusing in. This is called saturation.[3]
If the external partial pressure of the gas (in the lungs) is then reduced, more gas will diffuse out than in. This is a condition known as supersaturation. The gas will not necessarily form bubbles in the solvent at this stage, but supersaturation is necessary for bubble growth.[3]
Tissue half times
If an exponential uptake of gas is assumed, which is a good approximation of experimental values for diffusion in non-living homogenous materials, half time of a tissue is the time it takes for the tissue to take up or release 50% of the difference in dissolved gas capacity at a changed partial pressure. For each consecutive half time the tissue will take up or release half again of the cumulative difference in the sequence ½, ¾, 7/8, 15/16, 31/32, 63/64 etc. The number of half times chosen to assume full saturation depends on the decompression model, and typically ranges from 4 (93.75%) to 6 (98.44%).[19][20] Tissue compartment half times range from 1 minute to at least 720 minutes.[21]
- For example: A 5 minute tissue will be 50% saturated in 5 minutes, 75% in 10 minutes, 87.5% in 15 minutes and for practical purposes, saturated in about 30 minutes (98.44% saturated at 6 half times)
A specific tissue compartment will have different half times for gases with different solubilities and diffusion rates. This model may not adequately describe the dynamics of outgassing if gas phase bubbles are present.[22][23]
Outgassing of tissues
Gas remains dissolved in the tissues until the partial pressure of that gas in the lungs is reduced sufficiently to cause a concentration gradient with the blood at a lower concentration than the relevant tissues. A lowered partial pressure in the lungs will result in more gas diffusing out of the blood into the lung gas and less from the lung gas into the blood. A similar situation occurs between the blood and each tissue. As the concentration in the blood drops below the concentration in the adjacent tissue, the gas will diffuse out of the tissue into the blood, and will then be transported back to the lungs where it will diffuse into the lung gas and then be eliminated by exhalation. If the ambient pressure reduction is limited, this desaturation will take place in the dissolved phase, but if the ambient pressure is lowered sufficiently, bubbles may form and grow, both in blood and other supersaturated tissues.[3]
When the gas in a tissue is at a concentration where more diffuses out than in the tissue is said to be supersaturated with that gas relative to the surrounding tissues. Supersaturation can also be defined as when the combined partial pressures of gases dissolved in a tissue exceeds the total ambient pressure on the tissue,[24] and there is a theoretical possibility of bubble formation or growth.[3]
.svg.png)
Inherent unsaturation
There is a metabolic reduction of total gas pressure in the tissues.[25] The sum of partial pressures of the gas that the diver breathes must necessarily balance with the sum of partial pressures in the lung gas. In the alveoli the gas has been humidified by a partial pressure of approximately 63 mbar (47 mmHg) and has gained about 55 mbar (41 mmHg) carbon dioxide from the venous blood. Oxygen has also diffused into the arterial blood, reducing the partial pressure of oxygen in the alveoli by about 67 mbar(50 mmHg) As the total pressure in the alveoli must balance with the ambient pressure, this dilution results in an effective partial pressure of nitrogen of about 758 mb (569 mmHg) in air at normal atmospheric pressure.[25]
At a steady state, when the tissues have been saturated by the inert gases of the breathing mixture, metabolic processes reduce the partial pressure of the less soluble oxygen and replace it with carbon dioxide, which is considerably more soluble in water. In the cells of a typical tissue, the partial pressure of oxygen will drop to around 13 mbar (10 mmHg), while the partial pressure of carbon dioxide will be about 65 mbar (49 mmHg). The sum of these partial pressures (water, oxygen, carbon dioxide and nitrogen) comes to roughly 900 mbar (675 mmHg), which is some 113 mbar (85 mmHg) less than the total pressure of the respiratory gas. This is a significant saturation deficit, and it provides a buffer against supersaturation and a driving force for dissolving bubbles.[25]
Experiments suggest that the degree of unsaturation increases linearly with pressure for a breathing mixture of fixed composition, and decreases linearly with fraction of inert gas in the breathing mixture.[26] As a consequence, the conditions for maximising the degree of unsaturation are a breathing gas with the lowest possible fraction of inert gas – i.e. pure oxygen, at the maximum permissible partial pressure. This saturation deficit is also referred to as the "Oxygen window".[27] or partial pressure vacancy.[28]
Bubble formation, growth and elimination
The location of micronuclei or where bubbles initially form is not known.[29] Heterogeneous nucleation and tribonucleation are considered the most likely mechanism for bubble formation. Homogeneous nucleation requires much greater pressure differences than experienced in decompression.[29] The spontaneous formation of nanobubbles on hydrophobic surfaces is a possible source of micronuclei, but it is not yet clear if these can grow to symptomatic dimensions as they are very stable.[29]
The incorporation of bubble formation and growth mechanisms in decompression models may make the models more biophysical and allow better extrapolation.[29]
Flow conditions and perfusion rates are dominant parameters in competition between tissue and circulation bubbles, and between multiple bubbles, for dissolved gas for bubble growth.[29]
Bubble mechanics
Equilibrium of forces on the surface is required for a bubble to exist.[30] These are:
- Ambient pressure, exerted on the outside of the surface, acting inwards[30]
- Pressure due to tissue distortion, also on the outside and acting inwards[30]
- Surface tension of the liquid at the interface between the bubble and the surroundings. This is along the surface of the bubble, so the resultant acts towards the centre of curvature. This will tend to squeeze the bubble, and is more severe for small bubbles as it is an inverse function of the radius.[30]
- The resulting forces must be balanced by the pressure on the inside of the bubble. This is the sum of the partial pressures of the gases inside due to the net diffusion of gas to and from the bubble.[30]
- The force balance in the bubble may be modified by a layer of surface active molecules which can stabilise a microbubble at a size where surface tension on a clean bubble would cause it to collapse rapidly.[30]
- This surface layer may vary in permeability, so that if the bubble is compressed it may become impermeable to diffusion at sufficient compression.[30]
If the solvent outside the bubble is saturated or unsaturated, the partial pressure will be less than in the bubble, and the surface tension will be increasing the internal pressure in direct proportion to surface curvature, providing a pressure gradient to increase diffusion out of the bubble, effectively "squeezing the gas out of the bubble", and the smaller the bubble the faster it will get squeezed out. A gas bubble can only grow at constant pressure if the surrounding solvent is sufficiently supersaturated to overcome the surface tension or if the surface layer provides sufficient reaction to overcome surface tension.[30]
Clean bubbles that are sufficiently small will collapse due to surface tension if the supersaturation is low. Bubbles with semipermeable surfaces will either stabilise at a specific radius depending on the pressure, the composition of the surface layer, and the supersaturation, or continue to grow indefinitely, if larger than the critical radius.[31]
Bubble nucleation
Bubble formation occurs in the blood or other tissues. One of the hypothetical loci of bubble nucleation is in crevices in macromolecules.[32]
A solvent can carry a supersaturated load of gas in solution. Whether it will come out of solution in the bulk of the solvent to form bubbles will depend on a number of factors. Something which reduces surface tension, or adsorbs gas molecules, or locally reduces solubility of the gas, or causes a local reduction in static pressure in a fluid may result in a bubble nucleation or growth. This may include velocity changes and turbulence in fluids and local tensile loads in solids and semi-solids. Lipids and other hydrophobic surfaces may reduce surface tension (blood vessel walls may have this effect). Dehydration may reduce gas solubility in a tissue due to higher concentration of other solutes, and less solvent to hold the gas.[33]
Another theory presumes that microscopic bubble nuclei always exist in aqueous media, including living tissues. These bubble nuclei are spherical gas phases that are small enough to remain in suspension yet strong enough to resist collapse, their stability being provided by an elastic surface layer consisting of surface-active molecules which resists the effect of surface tension.[34]
Bubble growth
Once a micro-bubble forms it may continue to grow if the tissues are still supersaturated. As the bubble grows it may distort the surrounding tissue and cause damage to cells and pressure on nerves resulting in pain, or may block a blood vessel, cutting off blood flow and causing hypoxia in the tissues normally perfused by the vessel.[35] Bubbles can also damage the vascular endothelium through ischemia and reperfusion, physical contact with the endothelium or by physical deformation. This damage may release endothelial membrane microparticles.[36]
If a bubble or an object exists which collects gas molecules this collection of gas molecules may reach a size where the internal pressure exceeds the combined surface tension and external pressure and the bubble will grow.[37] If the solvent is sufficiently supersaturated, the diffusion of gas into the bubble will exceed the rate at which it diffuses back into solution, and if this excess pressure is greater than the pressure due to surface tension the bubble will continue to grow. When a bubble grows, the surface tension decreases, and the interior pressure drops, allowing gas to diffuse in faster, and diffuse out slower, so the bubble grows or shrinks in a positive feedback situation. The growth rate is reduced as the bubble grows because the surface area increases as the square of the radius, while the volume increases as the cube of the radius. If the external pressure is reduced due to reduced hydrostatic pressure during ascent, the bubble will also grow, and conversely,an increased external pressure will cause the bubble to shrink, but may not cause it to be eliminated entirely if a compression-resistant surface layer exists.[37]
The Variable Permeability Model ordering hypothesis states that nuclei are neither created nor totally eliminated during the pressure cycle, and the initial ordering according to size is preserved. Therefore, each bubble count is determined by the properties and behaviour of a nominal "critical" nucleus which is at the threshold of bubble-formation – all larger nuclei will form bubbles, and all smaller nuclei will not.[30]
Bubble distribution
Decompression bubbles appear to form mostly in the systemic capillaries where the gas concentration is highest, often those feeding the veins draining the active limbs. They do not generally form in the arteries provided that ambient pressure reduction is not too rapid, as arterial blood has recently had the opportunity to release excess gas into the lungs. The bubbles carried back to the heart in the veins may be transferred to the systemic circulation via a patent foramen ovale in divers with this septal defect, after which there is a risk of occlusion of capillaries in whichever part of the body they end up in.[38]
Bubbles are also known to form within other tissues, where they may cause damage leading to symptoms of decompression sickness. This damage is likely to be caused by mechanical deformation and stresses on the cells rather than local hypoxia, which is an assumed mechanism in the case of gas embolism of the capillaries.[39]
Bubble elimination
Bubbles which are carried back to the heart in the veins will normally pass into the right side of the heart, and from there they will normally enter the pulmonary circulation and eventually pass through or be trapped in the capillaries of the lungs, which are around the alveoli and very near to the respiratory gas, where the gas will diffuse from the bubbles though the capillary and alveolar walls into the gas in the lung. If the number of lung capillaries blocked by these bubbles is relatively small, the diver will not display symptoms, and no tissue will be damaged (lung tissues are adequately oxygenated by diffusion).[4]
The bubbles which are small enough to pass through the lung capillaries may be small enough to be dissolved due to a combination of surface tension and diffusion to a lowered concentration in the surrounding blood, though the Varying Permeability Model nucleation theory implies that most bubbles passing through the pulmonary circulation will lose enough gas to pass through the capillaries and return to the systemic circulation as recycled but stable nuclei.[40]
Bubbles which form within the tissues must be eliminated in situ by diffusion, which implies a suitable concentration gradient.[4]
Isobaric counterdiffusion (ICD)
Isobaric counterdiffusion is the diffusion of gases in opposite directions caused by a change in the composition of the external ambient gas or breathing gas without change in the ambient pressure. During decompression after a dive this can occur when a change is made to the breathing gas, or when the diver moves into a gas filled environment which differs from the breathing gas.[41]
While not strictly speaking a phenomenon of decompression, it is a complication that can occur during decompression, and that can result in the formation or growth of bubbles without changes in the environmental pressure. Two forms of this phenomenon have been described by Lambertsen:[42][41]
Superficial ICD (also known as Steady State Isobaric Counterdiffusion)[43] occurs when the inert gas breathed by the diver diffuses more slowly into the body than the inert gas surrounding the body.[42][41][43]
An example of this would be breathing air in an heliox environment. The helium in the heliox diffuses into the skin quickly, while the nitrogen diffuses more slowly from the capillaries to the skin and out of the body. The resulting effect generates supersaturation in certain sites of the superficial tissues and the formation of inert gas bubbles.[41]
Deep Tissue ICD (also known as Transient Isobaric Counterdiffusion)[43] occurs when different inert gases are breathed by the diver in sequence.[42] The rapidly diffusing gas is transported into the tissue faster than the slower diffusing gas is transported out of the tissue.[41]
This can occur as divers switch from a nitrogen mixture to a helium mixture (diffusivity of helium is 2.65 times faster than nitrogen),[41] or when saturation divers breathing hydreliox switch to a heliox mixture.[44]
There is another effect which can manifest as a result of the disparity in solubility between inert breathing gas diluents, which occurs in isobaric gas switches near the decompression ceiling between a low solubility gas (typically helium, and a higher solubility gas, typically nitrogen)[45][46]
An inner ear decompression model by Doolette and Mitchell suggests that a transient increase in gas tension after a switch from helium to nitrogen in breathing gas may result from the difference in gas transfer between compartments. If the transport of nitrogen into the vascular compartment by perfusion exceeds removal of helium by perfusion, while transfer of helium into the vascular compartment by diffusion from the perilymph and endolymph exceeds the counterdiffusion of nitrogen, this may result in a temporary increase in total gas tension, as the input of nitrogen exceeds the removal of helium, which can result in bubble formation and growth. This model suggests that diffusion of gases from the middle ear across the round window is negligible. The model is not necessarily applicable to all tissue types.[47]
Lambertsen made suggestions to help avoid ICD while diving:[42][41]
- If the diver is surrounded by or saturated with nitrogen, they should not breathe helium rich gases.
- Gas switches that involve going from helium rich mixtures to nitrogen rich mixtures would be acceptable, but changes from nitrogen to helium should include recompression.
However Doolette and Mitchell's more recent study of Inner Ear Decompression Sickness (IEDCS) shows that the inner ear may not be well-modelled by common (e.g. Bühlmann) algorithms. Doolette and Mitchell propose that a switch from a helium-rich mix to a nitrogen-rich mix, as is common in technical diving when switching from trimix to nitrox on ascent, may cause a transient supersaturation of inert gas within the inner ear and result in IEDCS.[47] They suggest that breathing-gas switches from helium-rich to nitrogen-rich mixtures should be carefully scheduled either deep (with due consideration to nitrogen narcosis) or shallow to avoid the period of maximum supersaturation resulting from the decompression. Switches should also be made during breathing of the largest inspired oxygen partial pressure that can be safely tolerated with due consideration to oxygen toxicity.[47]
A similar hypothesis to explain the incidence of IEDCS when switching from trimix to nitrox was proposed by Steve Burton, who considered the effect of the much greater solubility of nitrogen than helium in producing transient increases in total inert gas pressure, which could lead to DCS under isobaric conditions.[48]
Burton argues that effect of switching to Nitrox from Trimix with a large increase of nitrogen fraction at constant pressure has the effect of increasing the overall gas loading within particularly the faster tissues, since the loss of helium is more than compensated by the increase in nitrogen. This could cause immediate bubble formation and growth in the fast tissues. A simple rule for avoidance of ICD when gas switching at a decompression ceiling is suggested:[48]
- Any increase in gas fraction of nitrogen in the decompression gas should be limited to 1/5 of the decrease in gas fraction of helium.[48]
This rule has been found to successfully avoid ICD on hundreds of deep trimix dives.[48]
Ultrasonic bubble detection in decompression research
Doppler bubble detection equipment uses ultrasonic signals reflected from bubble surfaces to identify and quantify gas bubbles present in venous blood. This method was used by Dr Merrill Spencer of the Institute of Applied Physiology and Medicine in Seattle, who published a report in 1976 recommending that the then current no-decompression limits be reduced on the basis that large counts of venous gas bubbles were detected in divers exposed to the US Navy no-decompression limits. These non-symptomatic bubbles have become known as "silent bubbles", and are thought to contain nitrogen released from solution during ascent.[49] Doppler detection of venous bubbles has become an important tool in decompression research, partly because it allows a non-symptomatic endpoint for experimental work, and partly because the equipment has become relatively affordable for field surveys on divers conducting ordinary recreational, technical and professional dives.[50] Doppler bubble detection has also been used in saturation diving research.[51]
Doppler signals for bubbles are generally output as an audible signal, and may be graded according to the Spencer scale or the Kisman-Masurel scale. The Spencer scale was developed by Spencer and Johanson in 1974, and recognizes 5 grades of bubble signal against the background sounds of cardiac function: [52]
- Grade 0: No bubble signals detected
- Grade I: Occasional bubble signals detected - The majority of cardiac cycles are bubble-free
- Grade II: Many, but less than half of the cardiac cycles contain bubble signals
- Grade III: All cardiac cycles contain bubble signals, but they do not obscure the signals of cardiac activity
- Grade IV: Bubble signals are continuous, and obscure the sounds of normal heart function
The Kisman-Masurel scale is similar, and gives a more subtle gradation of bubbles, but is more difficult to rate proficiently. The Spencer scale has been more popular in practice. Grade categories are non-linear and cannot be averaged.[52]
Precordial monitoring of the pulmonary artery is the usual monitoring site, as it combines all the blood returning to the body before it goes to the lungs, so it is least likely to miss bubbles from a peripheral source, and is most compatible with the Spencer and K-M scales, as heart sounds are clearly audible. Other sites which have been used include the subclavian vein, carotid artery, femoral vein and inferior vena cava. Protocols for ultrasonic investigation of decompression bubbles are still in development, and may vary between researchers.[52]
Other methods of non-invasive bubble detection include two-dimensional echocardiography,[52] but Doppler appears to be more sensative and picks up smaller bubbles.[53]
Two dimensional imaging can provide a cross-sectional view along a single plane of all four chambers of the heart, and therefore, unlike Doppler, which assesses blood prior to primary filtration by the lungs, can also assess blood which will be circulated systemically. Echocardiography equipment has developed from bulky laboratory equipment to portable battery-powered with sufficient resolution suitable for field studies. Transthoracic echocardiography is suitable for the sampling used in decompression studies to identify highly reflective gas bubbles.[54] Detection of venous gas bubbles by ultrasound imaging is a sensitive, but not specific, predictor of adverse effects of decompression, similar to the published relationship between Doppler detected bubbles and decompression sickness.[55]
The correlation between Doppler-detected intravascular bubbles and decompression sickness is that almost all divers who developed DCS after a dive produced large numbers of bubbles, but even grade 3 or 4 bubbles could manifest without signs or symptoms of DCS, and grades 0, 1 and 2 bubbles are associated with very low risk. In a series of tests by Sawatsky, Grade 3 bubbles were associated with a 5% risk and Grade 4 with about 10% risk.[53] Bubbles may occur after exposures that have very good safety records. The utility of bubble detection is in assessing relative decompression stress.[54] The value of bubble detection in non-symptomatic divers, is that this can be used as a safer threshold for assessing acceptable decompression stress than the incidence of clinical symptoms for evaluating decompression algorithms.[54]
Decompression sickness and injuries
Intravascular bubbles cause clumping of red blood cells, platelets are used up, white blood cells activated, vascular permeability is increased. The gas in a bubble will equilibrate with the surrounding tissues and will therefore contain water vapor, oxygen, and carbon dioxide, as well as the inert gas. Vascular bubbles appear to form at the venous end of capillaries and pass through the veins to the right side of the heart, and thereafter are circulated to the lungs.[53]
Problems due to vascular decompression bubbles
Bubbles may be trapped in the lung capillaries, temporarily blocking them. If this is severe, the symptom called "chokes" may occur.[5]
If the diver has a patent foramen ovale (or a shunt in the pulmonary circulation), bubbles may pass through it and bypass the pulmonary circulation to enter the arterial blood. If these bubbles are not absorbed in the arterial plasma and lodge in systemic capillaries they will block the flow of oxygenated blood to the tissues supplied by those capillaries, and those tissues will be starved of oxygen. Moon and Kisslo (1988) concluded that "the evidence suggests that the risk of serious neurological DCI or early onset DCI is increased in divers with a resting right-to-left shunt through a PFO. There is, at present, no evidence that PFO is related to mild or late onset bends."[56]
Extravascular bubbles
Bubbles form within other tissues as well as the blood vessels.[5] Inert gas can diffuse into bubble nuclei between tissues. In this case, the bubbles can distort and permanently damage the tissue. As they grow, the bubbles may also compress nerves as they grow causing pain.[4][57]
Extravascular or autochthonous[a] bubbles usually form in slow tissues such as joints, tendons and muscle sheaths. Direct expansion causes tissue damage, with the release of histamines and their associated affects. Biochemical damage may be as important as, or more important than mechanical effects.[4][5][6]
Factors influencing uptake and elimination of dissolved gases and decompression risk
The exchange of dissolved gases between the blood and tissues is controlled by perfusion and to a lesser extent by diffusion, particularly in heterogeneous tissues. The distribution of blood flow to the tissues is variable and subject to a variety of influences. When the flow is locally high, that area is dominated by perfusion, and by diffusion when the flow is low. The distribution of flow is controlled by the mean arterial pressure and the local vascular resistance, and the arterial pressure depends on cardiac output and the total vascular resistance. Basic vascular resistance is controlled by the sympathetic nervous system, and metabolites, temperature, and local and systemic hormones have secondary and often localised effects, which can vary considerably with circumstances. Peripheral vasoconstriction in cold water decreases overall heat loss without increasing oxygen consumption until shivering begins, at which point oxygen consumption will rise, though the vasoconstriction can persist.[5]
Breathing gas composition
The composition of the breathing gas during pressure exposure and decompression is significant in inert gas uptake and elimination for a given pressure exposure profile. Breathing gas mixtures for diving will typically have a different gas fraction of nitrogen to that of air. The partial pressure of each component gas will differ to that of nitrogen in air at any given depth, and uptake and elimination of each inert gas component is proportional to the actual partial pressure over time. The two foremost reasons for use of mixed breathing gases are the reduction of nitrogen partial pressure by dilution with oxygen, to make Nitrox mixtures, primarily to reduce the rate of nitrogen uptake during pressure exposure, and the substitution of helium (and occasionally other gases) for the nitrogen to reduce the narcotic effects under high partial pressure exposure. Depending on the proportions of helium and nitrogen, these gases are called Heliox, if there is no nitrogen, or Trimix, if there is nitrogen and helium along with the essential oxygen.[58][59]
The inert gases used as substitutes for nitrogen have different solubility and diffusion characteristics in living tissues to the nitrogen they replace. For example, the most common inert gas diluent substitute for nitrogen is helium, which is significantly less soluble in living tissue,[60] but also diffuses faster due to the relatively small size and mass of the He atom in comparison with the N2 molecule.[61]
Body temperature and exercise
Blood flow to skin and fat are affected by skin and core temperature, and resting muscle perfusion is controlled by the temperature of the muscle itself. During exercise increased flow to the working muscles is often balanced by reduced flow to other tissues, such as kidneys spleen and liver.[5]
Blood flow to the muscles is lower in cold water, but exercise keeps the muscle warm and flow elevated even when the skin is chilled. Blood flow to fat normally increases during exercise, but this is inhibited by immersion in cold water. Adaptation to cold reduces the extreme vasoconstriction which usually occurs with cold water immersion.[5]
Variations in perfusion distribution do not necessarily affect respiratory inert gas exchange, though some gas may be locally trapped by changes in perfusion. Rest in a cold environment will reduce inert gas exchange from skin, fat and muscle, whereas exercise will increase gas exchange. Exercise during decompression can reduce decompression time and risk, providing bubbles are not present, but can increase risk if bubbles are present.[5]
Inert gas exchange is least favourable for the diver who is warm and exercises at depth during the ingassing phase, and rests and is cold during decompression.[5]
Other factors
Other factors which can affect decompression risk include oxygen concentration, carbon dioxide levels, body position, vasodilators and constrictors, positive or negative pressure breathing.[5] and dehydration (blood volume).[62]
Individual susceptibility to decompression sickness has components which can be attributed to a specific cause, and components which appear to be random. The random component makes successive decompressions a poor test of susceptibility.[5] Obesity and high serum lipid levels have been implicated by some studies as risk factors, and risk seems to increase with age.[63] Another study has also shown that older subjects tended to bubble more than younger subjects for reasons not yet known, but no trends between weight, body fat, or gender and bubbles were identified, and the question of why some people are more likely to form bubbles than others remains unclear.[64]
Saturation decompression
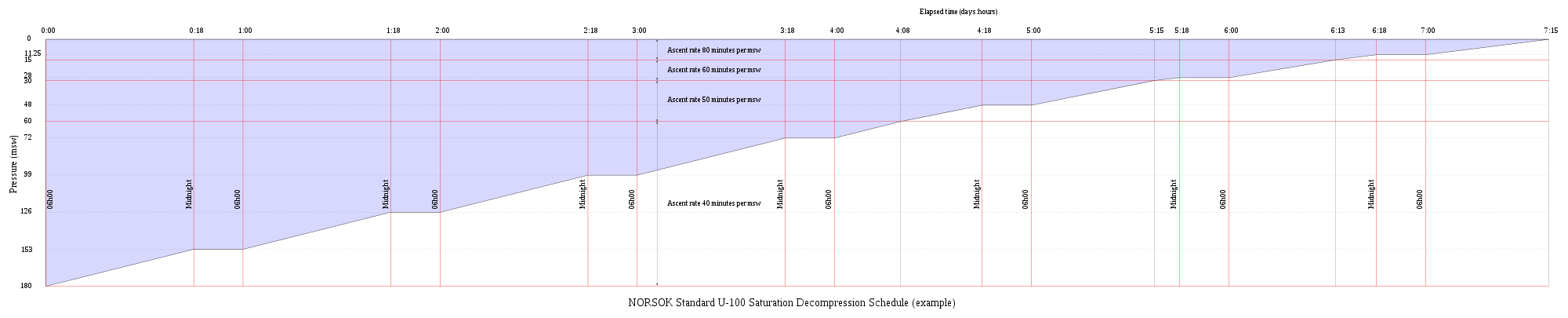
Saturation decompression is a physiological process of transition from a steady state of full saturation with inert gas at raised pressure to standard conditions at normal surface atmospheric pressure. It is a long process during which inert gases are eliminated at a very low rate limited by the slowest affected tissues, and a deviation can cause the formation of gas bubbles which can produce decompression sickness. Most operational procedures rely on experimentally derived parameters describing a continuous slow decompression rate, which may depend on depth and gas mixture.[65]
In saturation diving all tissues are considered saturated and decompression which is safe for the slowest tissues will theoretically be safe for all faster tissues in a parallel model. Direct ascent from air saturation at approximately 7 msw produces venous gas bubbles but not symptomatic DCS. Deeper saturation exposures require decompression to saturation schedules.[66]
The safe rate of decompression from a saturation dive is controlled by the partial pressure of oxygen in the inspired breathing gas.[67] The inherent unsaturation due to the oxygen window allows a relatively fast initial phase of saturation decompression in proportion to the oxygen partial pressure and then controls the rate of further decompression limited by the half-time of inert gas elimination from the slowest compartment.[68] However, some saturation decompression schedules specifically do not allow an decompression to start with an upward excursion.[69] Neither the excursions nor the decompression procedures currently in use (2016) have been found to cause decompression problems in isolation, but there appears to be significantly higher risk when excursions are followed by decompression before non-symptomatic bubbles resulting from excursions have totally resolved. Starting decompression while bubbles are present appears to be the significant factor in many cases of otherwise unexpected decompression sickness during routine saturation decompression.[70]
Application of a bubble model in 1985 allowed successful modelling of conventional decompressions, altitude decompression, no-stop thresholds, and saturation dives using one setting of four global nucleation parameters.[71]
Research continues on saturation decompression modelling and schedule testing. In 2015 a concept named Extended Oxygen Window was used in preliminary tests for a modified saturation decompression model. This model allows a faster rate of decompression at the start of the ascent to utilise the inherent unsaturation due to metabolic use of oxygen, followed by a constant rate limited by oxygen partial pressure of the breathing gas. The period of constant decompression rate is also limited by the allowable maximum oxygen fraction, and when this limit is reached, decompression rate slows down again as the partial pressure of oxygen is reduced. The procedure remains experimental as of May 2016. The goal is an acceptably safe reduction of overall decompression time for a given saturation depth and gas mixture.[65]
References
- ↑ Wienke, B.R. "Decompression theory" (PDF). Retrieved 9 February 2016.
- 1 2 3 4 US Navy 2008, Vol 1 Chpt. 3 Sec. 9.3
- 1 2 3 4 5 6 7 8 9 10 Huggins 1992, chpt. 1
- 1 2 3 4 5 Stephenson, Jeffrey (2016). "Pathophysiology, treatment and aeromedical retrieval of SCUBA – related DCI". Journal of Military and Veterans' Health. Australasian Military Medicine Association. 17 (3). ISSN 1839-2733.
- 1 2 3 4 5 6 7 8 9 10 11 Vann, R.D.(ed) (1989), The Physiological basis of decompression: an overview. pp1-10, Proceedings of the thirty-eighth undersea and hyperbaric medical society workshop, Undersea and Hyperbaric Medical Society, Bethesda, Maryland. http://archive.rubicon-foundation.org/6853
- 1 2 Kitano, Motoo (1995). "Pathological Aspects of Decompression Sicknes". 南太平洋海域調査研究報告=Occasional papers, Volume 25. 鹿児島大学. pp. 47–59. Retrieved 8 March 2016.
- ↑ Young, C.L.; Battino, R.; Clever, H.L. (1982). "The solubility of gases in liquids" (PDF). Retrieved 9 February 2016.
- ↑ John W. Hill, Ralph H. Petrucci, General Chemistry, 2nd edition, Prentice Hall, 1999.
- ↑ P. Cohen, ed. (1989). The ASME handbook on Water Technology for Thermal Power Systems. The American Society of Mechanical Engineers. p. 442.
- ↑ Henry, W. (1803). "Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures". Phil. Trans. R. Soc. Lond. 93: 29–274. doi:10.1098/rstl.1803.0004.
- ↑ Kasture, A.V. (October 2008). "5. Solubility of pharmaceiticals: Factors affecting solubility". Pharmaceutical Chemistry - I. Pragati Books Pvt. Ltd. p. 5.3. ISBN 9788185790121. Retrieved 7 March 2016.
- 1 2 Dueker, Christopher W (1985) [Rev. ed. of Medical aspects of sport diving, published 1970]. Scuba Diving in Safety and Health. Menlo Park, CA: Diving Safety Digest. p. 181. ISBN 0-9614638-0-5.
- ↑ "Diffusion (definition)". Biology online. Retrieved 7 March 2016.
- ↑ Burton, Steve (December 2004). "Isobaric Counter Diffusion". ScubaEngineer. Retrieved 3 February 2011.
- ↑ Huggins 1992, chpt. 9-page 6
- ↑ "15: Mixed gas and oxygen diving". The Noaa Diving Manual: Diving for Science and Technology (illustrated ed.). DIANE Publishing. 1992. p. 15.1. ISBN 9781568062310. Retrieved 8 March 2016.
- ↑ Hills, Brian A (1978). "Effect of decompression per se on nitrogen elimination". J Appl Physiol. 45 (6): 916–921. PMID 730597. Retrieved 31 October 2011.
- 1 2 Pittman, RN (2011). "Chapter 2: The Circulatory System and Oxygen Transport". Regulation of Tissue Oxygenation. San Rafael (CA): Morgan & Claypool Life Sciences.
- ↑ Huggins 1992, chpt. 2
- ↑ Bookspan, Jolie (June 2005). "Are Tissue Halftimes Real?". DAN Mediucal articles. Divers Alert Network. Retrieved 8 March 2016.
- ↑ Yount 1991, p. 137.
- ↑ Wienke, Bruce R. (1990). Michael A. Lang and Glen H. Egstrom, eds. "Phase dynamics and diving" (PDF). Proceedings of the AAUS Biomechanics of Safe Ascents Workshop. Costa Mesa CA.: American Academy of Underwater Science. pp. 13–29. Retrieved 8 March 2016.
- ↑ Yount, David E. (1990). Michael A. Lang and Glen H. Egstrom, eds. "The physics of bubble formation" (PDF). Proceedings of the AAUS Biomechanics of Safe Ascents Workshop. Costa Mesa CA.: American Academy of Underwater Science. pp. 13–29. Retrieved 8 March 2016.
- ↑ Huggins 1992, chpt. 1 page 7
- 1 2 3 Hills, Brian A (1978). "A fundamental approach to the prevention of decompression sickness". South Pacific Underwater Medicine Society Journal. 8 (2): 20–47. ISSN 0813-1988. OCLC 16986801. Retrieved 31 October 2011.
- ↑ Wienke 2002, p. 10
- ↑ Behnke, Albert R (1967). "The isobaric (oxygen window) principle of decompression". Trans. Third Marine Technology Society Conference, San Diego. The New Thrust Seaward. Washington DC: Marine Technology Society. Retrieved 19 June 2010.
- ↑ Van Liew, Hugh D; Conkin, J; Burkard, ME (1993). "The oxygen window and decompression bubbles: estimates and significance". Aviation, Space, and Environmental Medicine. 64 (9): 859–65. ISSN 0095-6562. PMID 8216150.
- 1 2 3 4 5 Papadopoulou, Virginie; Robert J. Eckersley; Costantino Balestra; Thodoris D. Karapantsios; Meng-Xing Tang (2013). "A critical review of physiological bubble formation in hyperbaric decompression". Advances in Colloid and Interface Science. Elsevier (191–192): 22–30.
- 1 2 3 4 5 6 7 8 9 Yount 1991, p. 131.
- ↑ Yount 1991, p. 132.
- ↑ Hills BA (March 1992). "A hydrophobic oligolamellar lining to the vascular lumen in some organs". Undersea Biomed Res. 19 (2): 107–20. PMID 1561717. Retrieved 31 October 2011.
- ↑ Tikuisis, P (1993). "Theoretical considerations for in vivo nucleation of bubbles". Abstract of the Undersea and Hyperbaric Medical Society, Inc. Annual Scientific Meeting held July 7–10, 1993. World Trade and Convention Centre, Halifax, Nova Scotia, Canada. Undersea and Hyperbaric Medical Society, Inc. Retrieved 8 March 2016.
- ↑ Yount 1991.
- ↑ Campbell, Ernest S. (1997). "Decompression Illness in Sports Divers: Part I". Medscape Orthopaedics & Sports Medicine eJournal, 1(5). Orange Beach, Ala.: Medscape Portals, Inc. Retrieved 14 March 2016.
- ↑ Madden, Leigh A.; Laden, Gerard (2009). "Gas bubbles may not be the underlying cause of decompression illness – The at-depth endothelial dysfunction hypothesis" (PDF). Medical Hypotheses. Elsevier. pp. 389–392. Retrieved 14 March 2016.
- 1 2 Yount, David E. (2002). "Decompression theory - Bubble models : Applying VPM to diving" (PDF). Diving Science:. Deep Ocean Diving. p. 8. Retrieved 11 March 2016.
- ↑ Vann, Richard D (1989). "An overview". The Physiological Basis of Decompression. Undersea and Hyperbaric Medical Society. Retrieved 12 March 2016.
- ↑ Wienke, B.R. "The elusive bubble". Retrieved 8 March 2016.
- ↑ Yount 1991, pp. 131,136.
- 1 2 3 4 5 6 7 Lambertson, Christian J (1989). Relations of isobaric gas counterdiffusion and decompression gas lesion diseases. In Vann, RD. "The Physiological Basis of Decompression". 38th Undersea and Hyperbaric Medical Society Workshop UHMS Publication Number 75(Phys)6-1-89. http://archive.rubicon-foundation.org/6853. Retrieved 10 January 2010.
- 1 2 3 4 Hamilton & Thalmann 2003, pp. 477–478.
- 1 2 3 D'Aoust, BG; White, R; Swanson, H; Dunford, RG; Mahoney, J (1982). "Differences in Transient and Steady State Isobaric Counterdiffusion". Report to the Office of Naval Research. http://archive.rubicon-foundation.org/4629. Retrieved 10 January 2010.
- ↑ Masurel, G; Gutierrez, N; Giacomoni, L (1987). "Hydrogen dive and decompression.". Abstract of the Undersea and Hyperbaric Medical Society, Inc. Annual Scientific Meeting held May 26–30, 1987. The Hyatt Regency Hotel, Baltimore, Maryland. Undersea and Hyperbaric Medical Society, Inc. Retrieved 14 March 2016.
- ↑ Partridge, Matthew. "Isobaric Inert Gas Counter diffusion" (PDF). Retrieved 14 March 2016.
- ↑ Burton, Steve (2011). "Isobaric Counter Diffusion How to avoid a Isobaric Counter Diffusion hit". ScubaEngineer.com. Retrieved 14 March 2016.
- 1 2 3 Doolette, David J; Mitchell, Simon J (June 2003). "Biophysical basis for inner ear decompression sickness". Journal of Applied Physiology. 94 (6): 2145–50. doi:10.1152/japplphysiol.01090.2002 (inactive 2015-01-01). PMID 12562679. Retrieved 10 January 2010.
- 1 2 3 4 Burton, Steve (December 2004). "Isobaric Counter Diffusion". ScubaEngineer. http://www.scubaengineer.com/isobaric_counter_diffusion.htm. Retrieved 10 January 2010.
- ↑ Huggins 1992, chpt. 4-page 6
- ↑ Dunford, RG; Wachholz, C; Fabus, S; Huggins, C; Mitchell, P; Bennett, PB (1991). "Doppler analysis of sport diver profiles.". Abstract of the Undersea and Hyperbaric Medical Society Annual Scientific Meeting held June 19–23, 1991 at San Diego Princess Resort, San Diego, CA. Undersea and Hyperbaric Medical Society. Retrieved 26 February 2016.
- ↑ Eftedal, 0. (1996-07-26). "Doppler measurements during saturation diving" (PDF). Report STF78 A961 27. Div. of Extreme Work Environment. Retrieved 16 October 2016.
- 1 2 3 4 Pollock, Neal W (2007). "Use of ultrasound in decompression research" (PDF). Diving and Hyperbaric Medicine: Volume 37, No 2. pp. 68–72. Retrieved 16 October 2016.
- 1 2 3 Sawatzky, David. "Doppler and Decompression Sickness" (PDF). pp. 173–175. Retrieved 16 October 2016.
- 1 2 3 Pollock, Neal W; Nishi, Ron Y (March 2014). "Ultrasonic detection of decompression-induced bubbles" (PDF). Diving and Hyperbaric Medicine Volume 44 No. 1. pp. 2–3. Retrieved 16 October 2016.
- ↑ Eftedal, OS; =Lydersen, S; Brubakk, AO (2007). "The relationship between venous gas bubbles and adverse effects of decompression after air dives." (PDF). Undersea and Hyperbaric Medicine, Volume 34, No. 2. Undersea and Hyperbaric Medical Society, Inc. pp. 99–105. Retrieved 16 October 2016.
- ↑ Moon, Richard E; Kisslo, Joseph (1998). "PFO and decompression illness: An update". South Pacific Underwater Medicine Society Journal. 28 (3). ISSN 0813-1988. OCLC 16986801. Retrieved 31 October 2011.
- ↑ Staff (May 2014). "Pathophysiology". Medscape Drugs & Diseases. Medscape. pp. Organ involvement associated with decompression sickness. Retrieved 8 March 2016.
- ↑ Brubakk, A. O.; T. S. Neuman (2003). Bennett and Elliott's physiology and medicine of diving (5th Rev ed.). United States: Saunders Ltd. p. 800. ISBN 0-7020-2571-2.
- ↑ Gernhardt, ML (2006). Lang, MA; Smith, NE, eds. "Biomedical and Operational Considerations for Surface-Supplied Mixed-Gas Diving to 300 FSW.". Proceedings of Advanced Scientific Diving Workshop. Washington, DC: Smithsonian Institution. Retrieved 21 October 2013.
- ↑ Scharlin, P.; Battino, R. Silla, E.; Tuñón, I.; Pascual-Ahuir, J. L. (1998). "Solubility of gases in water: Correlation between solubility and the number of water molecules in the first solvation shell". Pure & Appl. Chem. 70 (10): 1895–1904. doi:10.1351/pac199870101895
- ↑ Clifford A. Hampel (1968). The Encyclopedia of the Chemical Elements. New York: Van Nostrand Reinhold. pp. 256–268. ISBN 0-442-15598-0.
- ↑ Williams, ST; Prior, F; Bryson, PJ (2005), Haematocrit change in recreational Scuba divers following single dive exposure. http://archive.rubicon-foundation.org/1691
- ↑ Mouret, GML (2006). "Obesity and diving.". Journal of the South Pacific Underwater Medicine Society. Victoria, Australia: South Pacific Underwater Medicine Society. Retrieved 8 March 2016.
- ↑ Bookspan, J (May 2003). "Detection of endogenous gas phase formation in humans at altitude". Medicine & Science in Sports & Exercise Suppl. 35 (5,): S164. doi:10.1097/00005768-200305001-00901. Retrieved 7 May 2012.
- 1 2 Kot, Jacek; Sicko, Zdzislaw; Doboszynski, Tadeusz (2015). "The Extended Oxygen Window Concept for Programming Saturation Decompressions Using Air and Nitrox" (PDF). PLoS ONE 10(6): e0130835. pp. 1–20. doi:10.1371/journal.pone.0130835. Retrieved 13 May 2016.
- ↑ Eckenhoff, R.G.; Osborne, SF; Parker, JW; Bondi, KR (1986). "Direct ascent from shallow air saturation exposures". Undersea and Hyperbaric Medical Society, Inc. PMID 3535200. Retrieved 5 April 2016.
- ↑ Vann, R. D. (March 1984). "Decompression from Saturation Dives". Proceedings of the 3rd annual Canadian Ocean Technology Congress. Toronto, Canada. pp. 175–186. Retrieved 5 April 2016.
- ↑ Doboszynski, T; Sicko, Z; Kot, J (2012). "Oxygen-driven decompression after air, nitrox, heliox and trimix saturation exposures". Journal of the Undersea and Hyperbaric Medical Society. Undersea and Hyperbaric Medicine, Inc. Retrieved 5 April 2016.
- ↑ Staff (April 2009). NORSOK Standard U-100 : Manned underwater operations (3rd ed.). Lysaker, Norway: Standards Norway.
- ↑ Flook, Valerie (2004). Excursion tables in saturation diving - decompression implications of current UK practice RESEARCH REPORT 244 (PDF). Aberdeen United Kingdom: Prepared by Unimed Scientific Limited for the Health and Safety Executive. ISBN 0 7176 2869 8. Retrieved 27 November 2013.
- ↑ Hoffman, D.C.; Yount, DE (1985). "Tiny bubble helium decompression tables.". Abstract of the Undersea and Hyperbaric Medical Society, Inc. Annual Scientific Meeting. Undersea and Hyperbaric Medical Society, Inc. Retrieved 5 April 2016.
Sources
- Huggins, Karl E. (1992). "Dynamics of decompression workshop". Course taught at the University of Michigan. Retrieved 10 January 2012.
- Wienke, Bruce R; O'Leary, Timothy R (13 February 2002). "Reduced gradient bubble model: Diving algorithm, basis and comparisons" (PDF). Tampa, Florida: NAUI Technical Diving Operations. Retrieved 25 January 2012.
- Yount, DE (1991). Hans-Jurgen, K; Harper Jr, DE, eds. "Gelatin, bubbles, and the bends". International Pacifica Scientific Diving..., (Proceedings of the American Academy of Underwater Sciences Eleventh Annual Scientific Diving Symposium held 25–30 September 1991. University of Hawaii, Honolulu, Hawaii). Retrieved 25 January 2012.
- Hamilton, Robert W; Thalmann, Edward D (2003). "10.2: Decompression Practice". In Brubakk, Alf O; Neuman, Tom S. Bennett and Elliott's physiology and medicine of diving (5th Revised ed.). United States: Saunders. pp. 455–500. ISBN 0-7020-2571-2. OCLC 51607923.
Further reading
- Ball, R; Himm, J; Homer, LD; Thalmann, ED (1995). "Does the time course of bubble evolution explain decompression sickness risk?". Undersea and Hyperbaric Medicine. 22 (3): 263–280. ISSN 1066-2936. PMID 7580767.
- Gerth, Wayne A; Doolette, David J. (2007). "VVal-18 and VVal-18M Thalmann Algorithm – Air Decompression Tables and Procedures". Navy Experimental Diving Unit, TA 01-07, NEDU TR 07-09. Retrieved 27 January 2012.
- Gribble, M. de G. (1960); A comparison of the High-Altitude and High-Pressure syndromes of decompression sickness, Brit. J. industrial Med., 1960, 17, 181.
- Hills. B. (1966); A thermodynamic and kinetic approach to decompression sickness. Thesis
- Lippmann, John; Mitchell, Simon (2005). Deeper into Diving (2nd ed.). Melbourne, Australia: J L Publications. ISBN 0-9752290-1-X.
- Powell, Mark (2008). Deco for Divers. Southend-on-Sea: Aquapress. ISBN 1-905492-07-3.


