Controlled ovarian hyperstimulation
Controlled ovarian hyperstimulation is a technique used in assisted reproduction involving the use of fertility medications to induce ovulation by multiple ovarian follicles.[1] These multiple follicles can be taken out by oocyte retrieval (egg collection) for use in in vitro fertilisation (IVF), or be given time to ovulate, resulting in superovulation which is the ovulation of a larger-than-normal number of eggs,[2] generally in the sense of at least two. When ovulated follicles are fertilised in vivo, whether by natural or artificial insemination, there is a very high risk of a multiple pregnancy.
In contrast, when referring to treating menstrual disorders, such as oligoovulation or anovulation, the preferred term is ovulation induction.[3] In this article, unless otherwise specified, hyperstimulation will refer to hyperstimulation as part of IVF.
Procedure
Response prediction
Response predictors determine the protocol for ovulation suppression as well as dosage of medication used for hyperstimulation. Response prediction based on ovarian reserve confers substantially higher live birth rates, lower total costs and more safety.[4]
It is commonly agreed not to exclude anyone from their first IVF attempt only on the basis of poor results on response predictors, as the accuracy of these tests can be poor for the prediction of pregnancy.[5]
Antral follicle count
The response to gonadotropins may be roughly approximated by antral follicle count (AFC), estimated by vaginal ultrasound, which in turn reflects how many primordial follicles there are in reserve in the ovary.[6]
The definition of "poor ovarian response" is the retrieval of less than 4 oocytes following a standard hyperstimulation protocol, that is, following maximal stimulation.[5][note 1] On the other hand, the term "hyper response" refers to the retrieval of more than 15 or 20 oocytes following a standard hyperstimulation protocol.[5] The cut-offs used to predict poor responders versus normal versus hyper-responders upon vaginal ultrasonography vary in the literature, with that of likely poor response varying between an AFC under 3 and under 12, largely resulting from various definitions of the size follicles to be called antral ones.[5]
The following table defines antral follicles as those about 2–8 mm in diameter:[6]
| Antral follicle count | Classification[6] | Approximate expected response[6] | Risks[6] | Pregnancy rates[6] | Recommendation[6] |
|---|---|---|---|---|---|
| Less than 4 | Extremely low | Very poor or none | Cancelled cycle expected | 0–7% with 1 oocyte[7] | Not attempt IVF |
| 4-7 | Low | Possibly/probably poor response | Higher than average rate of IVF cycle cancellation | 15%[7] | High doses of gonadotropin likely |
| 8-10 | Reduced | Lower than average | Higher than average rate of IVF cycle cancellation | Slightly reduced[6] | |
| 11-14 | Normal (but intermediate) | Sometimes low, but usually adequate | Slight increased risk for IVF cycle cancellation | Slightly reduced compared to the "best" group[6] | |
| 15-30 | Normal (good) | Excellent | Very low risk for IVF cycle cancellation. Some risk for ovarian overstimulation | Best overall as a group[6] with approx. 35%[7] | Low doses of gonadotropins |
| More than 30 | High | Likely high | Overstimulation and ovarian hyperstimulation syndrome | Very good overall as a group, but potential egg quality issues[6] | Low doses of gonadotropins |
The incidence of poor ovarian response in IVF ranges from 10 to 20%.[5] Older poor responders have a lower range of pregnancy rates compared with younger ones (1.5–12.7 versus 13.0–35%, respectively).[7] Also, the other way around, there is a lower prevalence of poor responders among young women compared to those of advancing age, with 50% of women aged 43– 44 years being poor responders.[5]
Other response predictors
- Circulating anti-Müllerian hormone (AMH) can predict excessive and poor response to ovarian stimulation. According to NICE guidelines of in vitro fertilization, an anti-Müllerian hormone level of less than or equal to 5.4 pmol/l (0.8 ng/mL) predicts a low response to ovarian hyperstimulation, while a level greater than or equal to 25.0 pmol/l (3.6 ng/mL) predicts a high response.[8] For predicting an excessive response, AMH has a sensitivity and specificity of 82% and 76%, respectively.[9] Overall it may be superior to AFC and basal FSH.[10] Tailoring the dosage of gonadotrophin administration to AMH level has been shown to reduce the incidence of excessive response and cancelled cycles.[5]
- Elevated basal Follicle stimulating hormone (FSH) levels imply a need of more ampoules of gonadotropins for stimulation, and have a higher cancellation rate because of poor response.[11] However, one study came to the result that this method by itself is worse than only AMH by itself, with live birth rate with AMH being 24%, compared with 18% with FSH.[5]
- Advanced maternal age causes decreased success rates in ovarian hyperstimulation. In ovarian hyperstimulation combined with IUI, women aged 38–39 years appear to have reasonable success during the first two cycles, with an overall live birth rate of 6.1% per cycle.[12] However, for women aged ≥40 years, the overall live birth rate is 2.0% per cycle, and there appears to be no benefit after a single cycle of COH/IUI.[12] It is therefore recommended to consider in vitro fertilization after one failed COH/IUI cycle for women aged ≥40 years.[12]
- Body mass index[13]
- Previous hyperstimulation experiences[13]
- Length of menstrual cycles, with shorter cycles being associated with poorer response.[5]
- Previous ovarian surgery.[5]
Hyperstimulation medications
FSH preparations
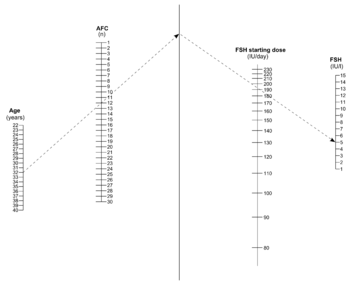
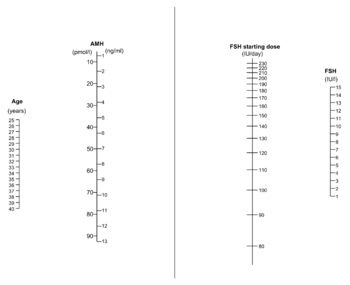
In most patients injectable gonadotropin preparations are used, usually FSH preparations. The clinical choice of gonadotrophin should depend on availability, convenience and costs.[14] The optimal dosage is mainly a trade-off between the pregnancy rate and risk of ovarian hyperstimulation syndrome.[13] A meta-analysis came to the result that the optimal daily recombinant FSH stimulation dose is 150 IU/day in presumed normal responders younger than 39 years undergoing IVF.[15] Compared with higher doses, this dose is associated with a slightly lower oocyte yield, but similar pregnancy rates and embryo cryopreservation rates.[15] For women predicted to have a poor response, there may not be any benefit to start at a higher FSH dosage than 150 IU per day.[5]
When used in medium dosage, a long-acting FSH preparation has the same outcome in regard to live birth rate and risk of ovarian hyperstimulation syndrome as compared to daily FSH. A long-acting FSH preparation may cause decreased live birth rates than using daily FSH when using low dosages (60 to 120 µg of corifollitropin alfa).[16]
Recombinant FSH (rFSH) appears to be equally effective in terms of live birth rate compared to any of the other types of gonadotropin preparations irrespective of the protocol used for ovulation suppression.[14]
Typically approximately 10 days of injections will be necessary.
Alternatives and complements to FSH
Administering recombinant hCG in addition to an FSH-preparation has no significant beneficial effect.[17]
A small number of underpowered randomized trials came to the result that use of clomifene in addition to gonadotropins results in similar live birth rate but with a reduction in the incidence of ovarian hyperstimulation syndrome.[14] A systematic review showed that using clomifene citrate in addition to low dose gonadotropin (in a GnRH antagonist protocol as described in the following section) resulted in a trend towards better pregnancy rates and a greater number of oocytes retrieved when compared with a standard high-dose FSH regime.[18] Such a protocol avails for using lower dosages of FSH-preparations, conferring lower costs per cycle, being particularly useful in cases where cost is a major limiting factor.[18]
Luteinizing hormone (LH) in addition to FSH has evidence of increased pregnancy rate, but not of live birth rate.[14] Using low dose human chorionic gonadotropin (hCG) to replace FSH during the late follicular phase in women undergoing hyperstimulation as part of IVF does not seem to reduce the chances of ongoing and clinical pregnancy, and likely results in an equivalent number of oocytes retrieved, but with less expenditure of FSH.[14] Administration of progestogen before hyperstimulation has evidence of improved pregnancy outcomes, while that of combined oral contraceptive pills before hyperstimulation has poorer pregnancy outcomes.[14]
Findings are conflicting, but metformin treatment as a complement in IVF cycles may reduce the risk of ovarian hyperstimulation syndrome and increase live birth rates.[19]
Suppression of spontaneous ovulation
When used in conjunction with in vitro fertilization (IVF), controlled ovarian hyperstimulation confers a need to avoid spontaneous ovulation, since oocyte retrieval of the mature egg from the fallopian tube or uterus is much harder than from the ovarian follicle. The main regimens to achieve ovulation suppression are:
- GnRH agonist administration given continuously before starting the gonadotropin hyperstimulation regimen. Physiologically, GnRH agonists are normally released in a cyclical fashion in the body to increase normal gonadotropin release, including luteinizing hormone that triggers ovulation, but continuous exogenous administration of GnRH agonists has the opposite effect of causing cessation of physiological gonadotropin production in the body.
- GnRH antagonist administration, which is typically administered in the mid-follicular phase in stimulated cycles after administration of gonadotropins and prior to triggering final maturation of oocytes. The GnRH antagonists that are currently licensed for use in fertility treatment are cetrorelix and ganirelix. In GnRH antagonist cycles, hyperstimulation medication is typically started on the second or third day of a previous natural menstruation.[20]
Agonist vs antagonist
Regarding pregnancy rate, choosing GnRH agonist protocol for a cycle is approximately as efficient as choosing GnRH antagonist protocol.[5][14] Still, the two protocols differ on a number of aspects:
- Practically, the timing of the hyperstimulation and the day of oocyte retrieval in a GnRH antagonist protocol needs to be timed after the spontaneous initiation of the previous menstrual cycle, while the schedule can be started at a time to meet practical needs in a GnRH agonist protocol.
- Regarding time per cycle, on the other hand, the cycle duration using GnRH antagonist protocol is typically substantially shorter than one using a standard long GnRH agonist protocol, potentially resulting in a higher amount of cycles in any given time period, which is beneficial for women with more limited time to become pregnant.[5]
- Regarding antral follicle count, with the GnRH antagonist protocol initial follicular recruitment and selection is undertaken by endogenous endocrine factors prior to starting the exogenous hyperstimulation. This results in a smaller number of growing follicles when compared with the standard long GnRH agonist protocol. This is an advantage in women expected to be high responders, thereby decreasing the risk of ovarian hyperstimulation syndrome.[5]
- Regarding subsequent final maturation induction, usage of GnRH agonist protocol necessitates subsequent usage of human chorionic gonadotropin (HCG or hCG) for this purpose, while usage of GnRH antagonist protocol also avails for subsequently using a GnRH agonist for final oocyte maturation. Using a GnRH agonist for final oocyte maturation rather than hCG results in an elimination of the risk of ovarian hyperstimulation syndrome, while having a delivery rate after IVF of approximately 6% less.[21]
Thus, in short, a GnRH antagonist protocol may be harder to schedule timewise but has shorter cycle lengths and less (or even eliminated) risk of ovarian hyperstimulation syndrome.
GnRH antagonist protocol has overall better results for expected poor and hyper-responders; A study of these protocols in women undergoing their first IVF and having a poor predicted response (by an AMH level below 5 pmol/l by DSL assay), using the GnRH antagonist protocol was associated with a substantial drop in cycle cancellation (odds ratio 0.20) and required fewer days of gonadotrophin stimulation (10 days versus 14 days) compared to GnRH agonist protocol.[5] Using GnRH antagonist protocol in high responders has been associated with significantly higher clinical pregnancy rates (62 versus 32%).[5]
The pregnancy rate is higher when a GnRH agonist was used in a long protocol as compared to a short or ultra-short GnRH agnoist protocol.[14] There is no evidence that stopping or reducing GnRH agonist administration at the start of gonadotropin administration results in a decrease in pregnancy rate.[14]
Monitoring
There is a concomitant monitoring, including frequently checking the estradiol level and, by means of gynecologic ultrasonography, follicular growth. Cycle monitoring by ultrasound plus serum estradiol compared to monitoring by ultrasound only does not increase live birth or pregnancy rates, but may be useful in preventing ovarian hyperstimulation syndrome (OHSS), and may therefore be a used in a subset of women to identify those at high risk of OHSS.[22]
Tracking or supervising the maturation of follicles is performed in order to timely schedule oocyte retrieval. Two-dimensional ultrasound is conventionally used. Automated follicle tracking does not appear to improve the clinical outcome of assisted reproduction treatment.[23]
Retrieval
When used in conjunction with IVF, ovarian hyperstimulation may be followed by final maturation of oocytes, using human chorionic gonadotropin (hCG), or a GnRH agonist if a GnRH antagonist protocol is used for ovulation suppression. A transvaginal oocyte retrieval is then performed just prior to when the follicles would rupture.
Coasting, which is ovarian hyperstimulation without induction of final maturation, does not significantly decrease the risk of ovarian hyperstimulation syndrome (OHSS), according to a Cochrane review of randomized trials.[14]
Risks
Ovarian hyperstimulation does not seem to be associated with an elevated risk of cervical cancer, nor with ovarian cancer or endometrial cancer when neutralizing the confounder of infertility itself.[24] Also, it does not seem to impact increased risk for breast cancer.[25]
Alternative
In vitro maturation is letting ovarian follicles mature in vitro, and with this technique ovarian hyperstimulation is not essential. Rather, oocytes can mature outside the body prior to fertilisation by IVF. Hence, gonadotropins does not need to be injected in the body, or at least a lower dose may be injected.[26] However, there is still not enough evidence to prove the effectiveness and safety of the technique.[26]
Notes
- ↑ Definition by the European Society of Human Reproduction and Embryology Consensus Conference
References
- ↑ TheFreeDictionary --> controlled ovarian hyperstimulation Retrieved on October 3, 2009
- ↑ Webster's New World College Dictionary » superovulation Retrieved on October 3, 2009
- ↑ Ovulation Problems and Infertility: Treatment of ovulation problems with Clomid and other fertility drugs. Advanced Fertility Center of Chicago. Gurnee & Crystal Lake, Illinois. Retrieved on Mars 7, 2010
- ↑ La Marca, A.; Sunkara, S. K. (2014). "Reply: The two sides of the individualization of controlled ovarian stimulation". Human Reproduction Update. 20 (4): 614–615. doi:10.1093/humupd/dmu014. ISSN 1355-4786.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 La Marca, A.; Sunkara, S. K. (2013). "Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: From theory to practice". Human Reproduction Update. 20 (1): 124–40. doi:10.1093/humupd/dmt037. PMID 24077980.
- 1 2 3 4 5 6 7 8 9 10 11 Antral Follicle Counts, Resting Follicles, Ovarian Volume and Ovarian Reserve. Testing of egg supply and predicting response to ovarian stimulation drugs Advanced Fertility Center of Chicago. Retrieved on October 2, 2009
- 1 2 3 4 Oudendijk, J. F.; Yarde, F.; Eijkemans, M. J. C.; Broekmans, F. J. M.; Broer, S. L. (2011). "The poor responder in IVF: Is the prognosis always poor? A systematic review". Human Reproduction Update. 18 (1): 1–11. doi:10.1093/humupd/dmr037. PMID 21987525.
- ↑ Fertility: assessment and treatment for people with fertility problems. NICE clinical guideline CG156 - Issued: February 2013
- ↑ Broer, S. L.; Dolleman, M.; Opmeer, B. C.; Fauser, B. C.; Mol, B. W.; Broekmans, F. J. M. (2010). "AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis". Human Reproduction Update. 17 (1): 46–54. doi:10.1093/humupd/dmq034. PMID 20667894.
- ↑ Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Luciano G. Nardo, M.D.a, Tarek A. Gelbaya, M.D.a, Hannah Wilkinson, MB.ChB.a, Stephen A. Roberts, Ph.D.b, Allen Yates, Ph.D.c, Phil Pemberton, B.A.c, Ian Laing, Ph.D.c. Fertility and Sterility. Volume 92, Issue 5, Pages 1586-1593 (November 2009)
- ↑ Useful Predictors of Ovarian Stimulation Response in Women Undergoing in vitro Fertilization. Jolande G. van der Stegea, Paul J.Q. van der Lindenb. Gynecol Obstet Invest 2001;52:43-46 doi:10.1159/000052939
- 1 2 3 Harris, I.; Missmer, S.; Hornstein, M. (2010). "Poor success of gonadotropin-induced controlled ovarian hyperstimulation and intrauterine insemination for older women". Fertility and Sterility. 94 (1): 144–148. doi:10.1016/j.fertnstert.2009.02.040. PMID 19394605.
- 1 2 3 He, M.; Zhao, L.; Powell, W. B. (2009). "Optimal control of dosage decisions in controlled ovarian hyperstimulation". Annals of Operations Research. 178: 223. doi:10.1007/s10479-009-0563-y.
- 1 2 3 4 5 6 7 8 9 10 Farquhar, C.; Rishworth, J. R.; Brown, J.; Nelen, W. L. M.; Marjoribanks, J. (2013). Brown, Julie, ed. "Assisted reproductive technology: an overview of Cochrane Reviews". The Cochrane Library. 8: CD010537. doi:10.1002/14651858.CD010537.pub2. PMID 23970457.
- 1 2 Sterrenburg, M. D.; Veltman-Verhulst, S. M.; Eijkemans, M. J. C.; Hughes, E. G.; MacKlon, N. S.; Broekmans, F. J.; Fauser, B. C. J. M. (2010). "Clinical outcomes in relation to the daily dose of recombinant follicle-stimulating hormone for ovarian stimulation in in vitro fertilization in presumed normal responders younger than 39 years: a meta-analysis". Human Reproduction Update. 17 (2): 184–96. doi:10.1093/humupd/dmq041. PMID 20843965.
- ↑ Pouwer, A. W.; Farquhar, C; Kremer, J. A. (2012). "Long-acting FSH versus daily FSH for women undergoing assisted reproduction". The Cochrane Library. 6: CD009577. doi:10.1002/14651858.CD009577.pub2. PMID 22696386.
- ↑ Cavagna, M.; Maldonado, L.; De Souza Bonetti, T.; de Almeida Ferreira Braga DP; Iaconelli Jr, A.; Borges Jr, E. (2010). "Supplementation with a recombinant human chorionic gonadotropin microdose leads to similar outcomes in ovarian stimulation with recombinant follicle-stimulating hormone using either a gonadotropin-releasing hormone agonist or antagonist for pituitary suppression". Fertility and Sterility. 94 (1): 167–172. doi:10.1016/j.fertnstert.2009.02.075. PMID 19342035.
- 1 2 Teixeira, D. M.; Martins, W. P. (2014). "The two sides of the individualization of controlled ovarian stimulation". Human Reproduction Update. 20 (4): 614–614. doi:10.1093/humupd/dmu013. ISSN 1355-4786.
- ↑ Sivalingam, V. N.; Myers, J.; Nicholas, S.; Balen, A. H.; Crosbie, E. J. (2014). "Metformin in reproductive health, pregnancy and gynaecological cancer: established and emerging indications". Human Reproduction Update. 20 (6): 853–868. doi:10.1093/humupd/dmu037. ISSN 1355-4786. PMID 25013215.
- ↑ Copperman, Alan B; Benadiva, Claudio (2013). "Optimal usage of the GnRH antagonists: a review of the literature". Reproductive Biology and Endocrinology. 11 (1): 20. doi:10.1186/1477-7827-11-20. ISSN 1477-7827.
- ↑ Humaidan, P.; Kol, S.; Papanikolaou, E.; Copenhagen GnRH Agonist Triggering Workshop Group (2011). "GnRH agonist for triggering of final oocyte maturation: time for a change of practice?". Hum. Reprod. Update. 17 (4): 510–524. doi:10.1093/humupd/dmr008. PMID 21450755.
- ↑ Kwan, Irene; Bhattacharya, Siladitya; Kang, Angela; Woolner, Andrea; Kwan, Irene (2014). "Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI)". Reviews. doi:10.1002/14651858.CD005289.pub3.
- ↑ Raine-Fenning, N.; Deb, S.; Jayaprakasan, K.; Clewes, J.; Hopkisson, J.; Campbell, B. (2010). "Timing of oocyte maturation and egg collection during controlled ovarian stimulation: a randomized controlled trial evaluating manual and automated measurements of follicle diameter". Fertility and Sterility. 94 (1): 184–188. doi:10.1016/j.fertnstert.2009.02.063. PMID 19342014.
- ↑ Siristatidis, C.; Sergentanis, T. N.; Kanavidis, P.; Trivella, M.; Sotiraki, M.; Mavromatis, I.; Psaltopoulou, T.; Skalkidou, A.; Petridou, E. T. (2012). "Controlled ovarian hyperstimulation for IVF: Impact on ovarian, endometrial and cervical cancer--a systematic review and meta-analysis". Human Reproduction Update. 19 (2): 105–123. doi:10.1093/humupd/dms051. PMID 23255514.
- ↑ Sergentanis, T. N.; Diamantaras, A. -A.; Perlepe, C.; Kanavidis, P.; Skalkidou, A.; Petridou, E. T. (2013). "IVF and breast cancer: A systematic review and meta-analysis". Human Reproduction Update. 20 (1): 106–123. doi:10.1093/humupd/dmt034. PMID 23884897.
- 1 2 Vejledning om kunstig befrugtning 2006 (Danish)
External links
- Antral Follicle Counts, Resting Follicles, Ovarian Volume and Ovarian Reserve Advanced Fertility Center of Chicago.