Membrane lipids
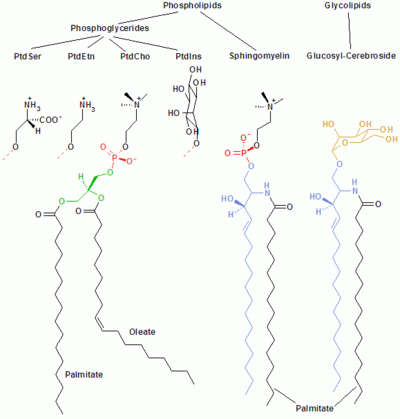
PtdCho - Phosphatidylcholine; PtdEtn - Phosphatidylethanolamine; PtdIns - Phosphatidylinositol; PtdSer - Phosphatidylserine.
Membrane lipids are a group of compounds (structurally similar to fats and oils) which form the double-layered surface of all cells (lipid bilayer). The three major classes of membrane lipids are phospholipids, glycolipids, and cholesterol. Lipids are amphiphilic: they have one end that is soluble in water ('polar') and an ending that is soluble in fat ('nonpolar'). By forming a double layer with the polar ends pointing outwards and the nonpolar ends pointing inwards membrane lipids can form a 'lipid bilayer' which keeps the watery interior of the cell separate from the watery exterior. The arrangements of lipids and various proteins, acting as receptors and channel pores in the membrane, control the entry and exit of other molecules and ions as part of the cell's metabolism. In order to perform physiological functions, membrane proteins are facilitated to rotate and diffuse laterally in two dimensional expanse of lipid bilayer by the presence of a shell of lipids closely attached to protein surface, called annular lipid shell.
Biological Roles
The bilayer formed by membrane lipids serves as a containment unit of a living cell. Membrane lipids also form a matrix in which membrane proteins reside. Historically lipids were thought to merely serve a structural role. Functional roles of lipids are in fact many: They serve as regulatory agents in cell growth and adhesion. They participate in the biosynthesis of other biomolecules. They can serve to increase enzymatic activities of enzymes.[1]
Non-bilayer forming lipid like monogalactosyl diglyceride(MGDG) predominates the bulk lipids in thylakoid membranes, which when hydrated alone, forms reverse hexagonal cylindrical phase. However, in combination with other lipids and carotenoids/chlorophylls of thylakoid membranes, they too conform together as lipid bilayers.[2]
Membrane lipid language
The membrane metabolites of polyunsaturated fatty acids (PUFAs) have an essential role in intercellular biochemical communications. Crawford (2010) in his chapter Long-chain polyunsaturated fatty acids in human brain evolution reported, with regard to the language of lipids, that the importance of the increased complexity of these lipids was brought about by aerobic metabolism, whereby the simple language of prokaryotes, with only a few words, was developed into a vocabulary of over 1,000 words of eukaryote cells.
About 500 million years ago, some nervous cells and some gut cells of vertebrates, migrated and specialized in a more complex nerrvous system: the brain, and in uptake and storage of iodocompounds: the follicular thyroid. In the PUFAs, the presence of a double bond between two carbons (or carbon-carbon double bond) provides them with the possibility of changing their molecular structure through enzymes such as phospholipases, cyclooxygenases and lipoxygenases, etc. The resulting substances, called eicosanoids: prostaglandins (PG), leukotrienes (LT), lipoxins and tromboxane (TX); and docosanoids: resolvins, protectins, and maresins, are powerful lipid mediators that produce specific actions in the organism; they organize inflammation, hemodynamic, immune response and the repair of tissue.
Many PUFAs cannot be synthesized by animal organisms and are considered essential, and therefore should be incorporated into diets. These are: linoleic acid (C18:2 n-6), omega-6 and alpha-linolenic (C18:3 n-3) omega-3, arachidonic acid (AA) - omega - 6 (C20: 4n-6), and docosahexaenoic acid (DHA) - omega -3 (C22:6n-3). These PUFAs are incorporated into the phospholipidic membrane of all the cells of an organism. In parallel, ectodermic cells, differentiated into neuronal cells, became the primitive nervous system and brain. Both these cells synthesized iodolipids, as novel words of the chemical lipid language developed among cell membranes during the evolution of life. These biochemical signals among cells, since contact and modification of membranes in multicellular organisms formed the bases of adaptation to terrestrial environments, and their alterations are important in the mechanism of apoptosis, carcinogenesis and degenerative diseases, as well as for understand some problems discussed regarding human evolution (as Aquatic ape hypothesis) [3] .[4][5][6][7]
Major classes
Phospholipids
Phospholipids and glycolipids consist of two long, nonpolar (hydrophobic) hydrocarbon chains linked to a hydrophilic head group.
The heads of phospholipids are phosphorylated and they consist of either:
- Glycerol (and hence the name phosphoglycerides given to this group of lipids), or
- Sphingosine (e.g. sphingomyelin and ceramide).
Glycolipids
The heads of glycolipids (glyco- stands for sugar) contain a sphingosine with one or several sugar units attached to it. The hydrophobic chains belong either to:
- two fatty acids (FA) - in the case of the phosphoglycerides, or
- one FA and the hydrocarbon tail of sphingosine - in the case of sphingomyelin and the glycolipids.
Galactolipids- monogalactosyl diglyceride(MGDG) and digalactosyl diglycreride(DGDG) form the predominant lipids in higher plant chloroplast thylakoid membranes; liposomal structures formed by total lipid extract of thylakoid membranes have been found sensitive to sucrose as it turns bilayers into micellar structures.[8]
Fatty acids
The fatty acids in phospho- and glycolipids usually contain an even number, typically between 14 and 24, of carbon atoms, with 16- and 18-carbon being the most common. FAs may be saturated or unsaturated, with the configuration of the double bonds nearly always cis. The length and the degree of unsaturation of FAs chains have a profound effect on membranes' fluidity. Plant thylakoid membranes maintain high fluidity, even at relatively cold environmental temperatures, due the abundance of 18-carbon fatty acyl chains with three double bonds, linolenic acid, as has been revealed by 13-C NMR studies.[9]
Phosphoglycerides
In phosphoglycerides, the hydroxyl groups at C-1 and C-2 of glycerol are esterified to the carboxyl groups of the FAs. The C-3 hydroxyl group is esterified to phosphoric acid. The resulting compound, called phosphatidate, is the simplest phosphoglycerate. Only small amounts of phosphatidate are present in membranes. However, it is a key intermediate in the biosynthesis of the other phosphoglycerides.
Sphingolipids
Sphingosine is an amino alcohol that contains a long, unsaturated hydrocarbon chain. In sphingomyelin and glycolipids, the amino group of sphingosine is linked to FAs by an amide bond. In sphingomyelin the primary hydroxyl group of sphingosine is esterified to phosphoryl choline.
In glycolipids, the sugar component is attached to this group. The simplest glycolipid is cerebroside, in which there is only one sugar residue, either Glc or Gal. More complex glycolipids, such as gangliosides, contain a branched chain of as many as seven sugar residues.
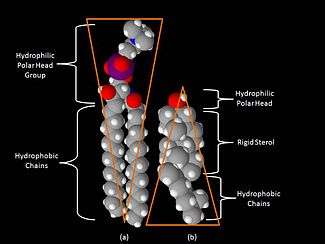
Sterols
The best known sterol is cholesterol, which is found in humans. Cholesterol also occurs naturally in other eukaryote cell membranes. Sterols have a hydrophobic four-membered fused ring rigid structure, and a small polar head group.
Cholesterol is bio-synthesised from mevalonate via a squalene cyclisation of terpenoids. Cell membranes require high levels of cholesterol - typically an average of 20% cholesterol in the whole membrane, increasing locally in raft areas up to 50% cholesterol (- % is molecular ratio).[10] It associates preferentially with sphingolipids (see diagram) in cholesterol-rich lipid rafts areas of the membranes in eukaryotic cells.[11] Formation of lipid rafts promotes aggregation of peripheral and transmembrane proteins including docking of SNARE and VAMP proteins[12] Phytosterols, such as sitosterol and stigmasterol, and Hopanoids serve a similar function in plants and prokaryotes.
See also
- Lipid bilayer
- Homeoviscous adaptation
- Membrane protein
- Annular lipid shell
- Protein-lipid interaction
References
- ↑ R. B. Gennis. Biomembranes - Molecular Structure and Function. Springer-Verlag, New York (1989).
- ↑ YashRoy R.C. (1990) Lamellar dispersion and phase separation of chloroplast membrane lipids by negative staining electron microscopy. Journal of Biosciences, vol. 15(2), pp. 93-98.https://www.researchgate.net/publication/230820037_Lamellar_dispersion_and_phase_separation_of_chloroplast_membrane_lipids_by_negative_staining_electron_microscopy?ev=prf_pub
- ↑ Venturi, S.; Donati, F.M.; Venturi, A.; Venturi, M. (2000). "Environmental Iodine Deficiency: A Challenge to the Evolution of Terrestrial Life?". Thyroid. 10 (8): 727–9. doi:10.1089/10507250050137851. PMID 11014322.
- ↑ Crawford, M. A.; Bloom, M.; Broadhurst, C. L.; Schmidt, W. F.; Cunnane, S. C.; Galli, C.; Gehbremeskel, K.; Linseisen, F.; Lloyd-Smith, J.; Parkington, J. (1999). "Evidence for the unique function of docosahexaenoic acid during the evolution of the modern hominid brain". Lipids. 34 Suppl: S39–S47. doi:10.1007/BF02562227. PMID 10419087.
- ↑ Venturi, S; Bégin ME (2010). "Thyroid Hormone, Iodine and Human Brain Evolution". In Cunnane S; Stewart K. Environmental Influences on Human Brain Evolution. John Wiley & Sons. pp. 105–124. ISBN 978-0-470-45268-4.
- ↑ Crawford MA (2010). "Long-Chain Polyunsaturated Fatty Acids in Human Brain Evolution". In Cunnane S; Stewart K. Environmental Influences on Human Brain Evolution. John Wiley & Sons. pp. 13–32. ISBN 978-0-470-45268-4.
- ↑ Venturi, Sebastiano (2014). "Iodine, PUFAs and Iodolipids in Health and Disease: An Evolutionary Perspective". Human Evolution-. 29 (1-3): 185–205. ISSN 0393-9375.
- ↑ YashRoy R.C. (1994) Destabilisation of lamellar dispersion of thylakoid membrane lipids by sucrose. Biochimica et Biophysica Acta, vol. 1212, pp. 129-133.https://www.researchgate.net/publication/15042978_Destabilisation_of_lamellar_dispersion_of_thylakoid_membrane_lipids_by_sucrose?ev=prf_pub
- ↑ YashRoy R.C. (1987) 13-C NMR studies of lipid fatty acyl chains of chloroplast membranes. Indian Journal of Biochemistry and Biophysics, vol. 24(6), pp. 177-178.https://www.researchgate.net/publication/230822408_13-C_NMR_studies_of_lipid_fatty_acyl_chains_of_chloroplast_membranes?ev=prf_pub
- ↑ de Meyer F, Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc Natl Acad Sci U S A 2009; 106: 3654-8.
- ↑ Chen, Heshun; Born, Ella; Mathur, Satya N.; Field, F. Jeffrey (December 1, 1993). "Cholesterol and sphingomyelin syntheses are regulated independently in cultured human intestinal cells, CaCo-2: role of membrane cholesterol and sphingomyelin content." (PDF). Journal of Lipid Research. American Society for Biochemistry and Molecular Biology. 34 (12): 2159–67. ISSN 0022-2275.
- ↑ Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis EMBO J 2001;20:2202-13.
External links
| Wikimedia Commons has media related to Membrane lipids. |
- Membrane lipids at the US National Library of Medicine Medical Subject Headings (MeSH)