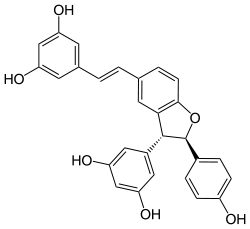
delta-Viniferin
 | |
| Names | |
|---|---|
| IUPAC name
5-{(E)-2-[(2R,3R)-3-(3,5-Dihydroxyphenyl)-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-5-yl]vinyl}-1,3-benzenediol | |
| Other names | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 552752 |
| PubChem | 637098 |
| |
| Properties | |
| C28H22O6 | |
| Molar mass | 454.47 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine (Vitis vinifera) leaves.[2] It is also found in plant cell cultures.[3] or in wine.[4] It can also be found in Rheum maximowiczii.[1]
It is a grapevine phytoalexin following stresses[2] like fungal infection (by Plasmopara viticola, the agent of downy mildew),[5] UV light irradiation or ozone treatment.[6]
Botryosphaeria obtusa, a pathogen responsible for the black dead arm disease of grapevine, has also been shown to be able to oxidise wood δ-resveratrol into delta-viniferin.[7]
In cell cultures, the use of methyl jasmonate and jasmonic acid as elicitors stimulates δ-viniferin biosynthesis.[8]
Delta-viniferin can also be produced from resveratrol by human PTGS1 (COX-1, cyclooxygenase-1)[9] or from trans-resveratrol and (−)-epsilon-viniferin by horseradish peroxidase.[10]
See also
References
- 1 2 Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O. K.; Ashurmetov, O. (2001). "Phenylbutanoids and stilbene derivatives of Rheum maximowiczii". Phytochemistry. 56 (4): 377–381. doi:10.1016/S0031-9422(00)00370-8. PMID 11249105.
- 1 2 Pezet, R.; Perret, C.; Jean-Denis, J. B.; Tabacchi, R.; Gindro, K.; Viret, O. (2003). "Δ-Viniferin, a Resveratrol Dehydrodimer: One of the Major Stilbenes Synthesized by Stressed Grapevine Leaves". Journal of Agricultural and Food Chemistry. 51 (18): 5488–5492. doi:10.1021/jf030227o. PMID 12926902.
- ↑ Waffo-Teguo, P.; Lee, D.; Cuendet, M.; Mérillon, J. M.; Pezzuto, J. M.; Kinghorn, A. D. (2001). "Two New Stilbene Dimer Glucosides from Grape (Vitisvinifera) Cell Cultures". Journal of Natural Products. 64 (1): 136–138. doi:10.1021/np000426r. PMID 11170689.
- ↑ Vitrac, X.; Bornet, A. L.; Vanderlinde, R.; Valls, J.; Richard, T.; Delaunay, J. C.; Mérillon, J. M.; Teissédre, P. L. (2005). "Determination of Stilbenes (δ-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, ε-viniferin) in Brazilian Wines". Journal of Agricultural and Food Chemistry. 53 (14): 5664–5669. doi:10.1021/jf050122g. PMID 15998130.
- ↑ Timperio, A. M.; d’Alessandro, A.; Fagioni, M.; Magro, P.; Zolla, L. (2012). "Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions". Plant Physiology and Biochemistry. 50 (1): 65–71. doi:10.1016/j.plaphy.2011.07.008. PMID 21821423.
- ↑ González-Barrio, R. O.; Beltrán, D.; Cantos, E.; Gil, M. A. I.; Espín, J. C.; Tomás-Barberán, F. A. (2006). "Comparison of Ozone and UV-C Treatments on the Postharvest Stilbenoid Monomer, Dimer, and Trimer Induction in Var. 'Superior' White Table Grapes". Journal of Agricultural and Food Chemistry. 54 (12): 4222–4228. doi:10.1021/jf060160f. PMID 16756350.
- ↑ Djoukeng, J. D. S.; Polli, S.; Larignon, P.; Abou-Mansour, E. (2009). "Identification of phytotoxins from Botryosphaeria obtusa, a pathogen of black dead arm disease of grapevine". European Journal of Plant Pathology. 124 (2): 303. doi:10.1007/s10658-008-9419-6.
- ↑ Santamaria, A. R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. (2011). "Effects of Elicitors on the Production of Resveratrol and Viniferins in Cell Cultures ofVitis viniferaL. Cv Italia". Journal of Agricultural and Food Chemistry. 59 (17): 9094–9101. doi:10.1021/jf201181n. PMID 21751812.
- ↑ Szewczuk, L. M.; Lee, S. H.; Blair, I. A.; Penning, T. M. (2005). "Viniferin Formation by COX-1: Evidence for Radical Intermediates during Co-oxidation of Resveratrol". Journal of Natural Products. 68 (1): 36–42. doi:10.1021/np049702i. PMID 15679314.
- ↑ Wilkens, A.; Paulsen, J.; Wray, V.; Winterhalter, P. (2010). "Structures of Two Novel Trimeric Stilbenes Obtained by Horseradish Peroxidase Catalyzed Biotransformation oftrans-Resveratrol and (−)-ε-Viniferin". Journal of Agricultural and Food Chemistry. 58 (11): 6754–6761. doi:10.1021/jf100606p. PMID 20455561.