Magnesium silicide
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Magnesium silicide | |
| Identifiers | |
| 22831-39-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 81111 |
| ECHA InfoCard | 100.041.125 |
| EC Number | 245-254-5 |
| PubChem | 89858 |
| |
| |
| Properties | |
| Mg2Si | |
| Molar mass | 76.69 g·mol−1 |
| Density | 1.988 g cm−3 |
| Melting point | 1,102 °C (2,016 °F; 1,375 K) |
| Hazards | |
| Main hazards | reacts with water to give silane |
| R-phrases | R23, R24, R25, R34 |
| Related compounds | |
| Other cations |
Calcium silicide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Magnesium silicide, Mg2Si, is an inorganic compound consisting of magnesium and silicon. As a powder magnesium silicide is dark blue or slightly purple in color.
Synthesis

It can be produced by heating silicon dioxide, SiO2, found in sand, with excess magnesium. The process first forms silicon metal and magnesium oxide, and, if an excess of magnesium is used, the silicide is formed:
- 2 Mg + SiO2 → 2 MgO + Si
If an excess of Mg is present, Mg2Si is formed from the reaction of the remaining magnesium with the silicon:
- 2 Mg + Si → Mg2Si
These reactions proceed exothermically.[1]
Uses
Magnesium silicide is used to create aluminium alloys of the 6000 series, containing up to approximately 1.5% Mg2Si. An alloy of this group can be age-hardened to form Guinier-Preston zones and a very fine precipitate, both resulting in increased strength of the alloy.[2]
Reactions

Magnesium silicide can be viewed as consisting of Si4− ions. As such it is reactive toward acids. Thus, when magnesium silicide is treated with hydrochloric acid, silane, SiH4 is produced:
- Mg2Si + 4 HCl → SiH4 + 2 MgCl2
Sulfuric acid can be used as well. These protonolysis reactions are typical of a Group 2 and Group 1 silicides.
Crystal Structure
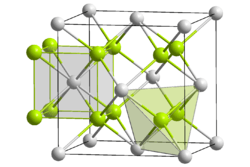
Mg2Si crystallizes in the antifluorite structure. In the face-centered cubic lattice Si centers occupy the corners and face-centered positions of the unit cell and Mg centers occupy eight tetrahedral sites in the interior of the unit cell. The coordination numbers of Si and Mg are eight and four, respectively.[3]
References
- ↑ P. Ehrlich "Alkaline Earth Metals" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 920.
- ↑ ASM Handbook, 10th Ed., Vol. 1, Properties and Selection: Non-ferrous Alloys and Special Purpose Materials, 1990, ASM International, Materials Park, Ohio.
- ↑ A. Kato et al. J. Phys: Condens. Matter 21 (2009) 205801.