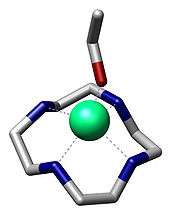
Macrocyclic ligand
In chemistry, a macrocyclic ligand is a macrocycle with three or more donor sites. Classic examples are crown ethers and porphyrins. [1] Macrocyclic ligands exhibit particularly high affinity for metal ions.
History
Porphyrins and phthalocyanines have long been recognized as potent ligands in coordination chemistry. In the 1960's the synthesis of macrocylic ligands received much attention, beginning with the synthesis of the "Curtis macrocycles."[2] Similarly, porphyrins, which feature 16-membered central rings, form in the absence of metal templates.

In 1967 a new series of macrocyclic compounds was reported by Pederson, who synthesized a number of cyclic polyethers or “crown” compounds with variety of ring sizes, a number of ether oxygens and substituent groups.[3] Shortly after crown compounds, cryptands, were reported by Lehn and co-workers.[4]
Macrocyclic effect
The macrocyclic effect is a term that describes the high affinity of metal cations for macrocyclic ligands, compared to their acyclic analogues.[5] [6] The high affinity of macrocyclic ligands is thought to be a combination of the entropic effect seen in the chelate effect, together with an additional energetic contribution that comes from the preorganized nature of the ligating groups (that is, no additional strains are introduced to the ligand on coordination).[7]
Synthesis
In general, macrocyclic complexes are synthesized by combining macrocyclic ligands and metal ions.[8]
In template reactions, macrocyclic ligands are generated in the presence of metal ions. In the absence of the metal ion, the same organic reactants produce different, often polymeric, products. The term is mainly used in coordination chemistry. The template effects emphasizes the pre-organization provided by the coordination sphere, although the coordination modifies the electronic properties (acidity, electrophilicity, etc.) of ligands. A challenge with templated synthesis of macrocyclic ligands is difficulting in removing the templating metal from the ligand.
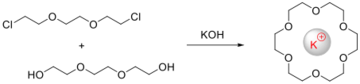
The course of the templated cyclization depends on the presence of metal ion, which is provide directional influence, therefore acting as a 'template' for the cyclization reaction. Phthalocyanine was the first macrocycle synthesis by template reaction, bearing strong structural resemblance to natural porphyrin systems. The metal ion may direct the condensation preferentially to cyclic rather than polymeric products (the kinetic template effect) or stabilize the macrocycle once formed (the thermodynamic template effect).
The size of the cation used as the template has proved to be of importance in directing the synthetic pathway for the Schiff base systems. The compatibility between the radius of the template cation and the “hole” of the macrocycle contributes to the effectiveness of the synthetic pathway and to the geometry of the resulting complex [9]
Uses and occurrence
Phthalocyanines, as their metal complexes, are arguably the most commercially useful complex of a macrocyclic ligand. They are uses as dyes and pigments.[10]
Macrocyclic ligands occur in many cofactors in proteins and enzymes. Of particular interest are tetraazamacrocycles.[11]
Heme, the active site in the hemoglobin (the protein in blood that transports oxygen), is a porphyrin containing iron. Chlorophyll, the green photosynthetic pigment found in plants, contains a chlorin ring. Vitamin B12 contains a corrin ring.
References
- ↑ http://goldbook.iupac.org/search.py?search_text=macrocyclic+ligand
- ↑ N.F. Curtis "Macrocyclic coordination compounds formed by condensation of metal-amine complexes with aliphatic carbonyl compounds" Coordination Chemistry Reviews, 1968 Volume 3, pp. 3-47. doi:10.1016/S0010-8545(00)80104-6
- ↑ Pedersen C J. Cyclic polyethers and their complexes with metal salts. J. Amer. Chem. Soc. 89:7017-36. 1967
- ↑ C. J. Pedersen and H. K. Frensdorff, Angew. Chem., Int. Ed. Engl. 1971, volume 11, p. 972.
- ↑ Cabbines, D. K.; Margerum, D. W. (1969). "Macrocyclic effect on stability of copper(II) tetramine complexes". J. Am. Chem. Soc. 91 (23): 6540–6541. doi:10.1021/ja01051a091.
- ↑ Melson, G.A., Ed. (1979). Coordination Chemistry of Macrocyclic Compounds. New York: Plenum Press. ISBN 0-306-40140-1.
- ↑ Weller, M. et al (2014), Inorganic Chemistry, 6th edition, Oxford University Press
- ↑ L.F Lindloy, The Chemistry of Macrocylic Ligand Complexes, Cambridge University Press, 1989, 20-50 ISBN 0-521-25261-X
- ↑ V. Alexander , Design and Synthesis of Macrocyclic Ligands and Their Complexes of Lanthanides and Actinides, Chem. Rev. 1995, 95, 273-342
- ↑ Milgrom, L.R (1997). The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds. New York: Oxford University Press. ISBN 0-19-855380-3. (hardbound) ISBN 0-19-855962-3 (pbk.)
- ↑ S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.