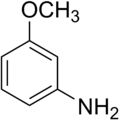
m-Anisidine
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3-Methoxyaniline | |||
| Other names
m-Anisidine (no longer recommended[1]) | |||
| Identifiers | |||
| 536-90-3 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 13869480 | ||
| ECHA InfoCard | 100.007.867 | ||
| EC Number | 208-651-4 | ||
| UN number | 2431 | ||
| |||
| |||
| Properties[2] | |||
| C7H9NO | |||
| Molar mass | 123.15 g/mol | ||
| Appearance | pale yellow oily liquid | ||
| Density | 1.096 (20 °C), liquid | ||
| Melting point | < 0 °C (32 °F; 273 K) | ||
| Boiling point | 251 °C (484 °F; 524 K) | ||
| Solubility | soluble in ethanol, diethyl ether, acetone, benzene | ||
| Refractive index (nD) |
1.5794 | ||
| Hazards | |||
| Flash point | > 122 °C (252 °F; 395 K) | ||
| 515 °C (959 °F; 788 K) | |||
| Related compounds | |||
| Related compounds |
o-Anisidine p-Anisidine | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
m-Anisidine one of the three isomers of anisidine. It is very poisonous for the blood leading to pink coloration of the skin and inner suffocation. Additionally its vapors are irritating to eyes, mucous membranes, the respiratory system and skin.
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 669. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The names ‘toluidine’, ‘anisidine’, and ‘phenetidine’ for which o-, m-, and p- have been used to distinguish isomers, and ‘xylidine’ for which numerical locants, such as 2,3-, have been used, are no longer recommended, nor are the corresponding prefixes ‘toluidine’, ‘anisidino’, ‘phenetidine’, and ‘xylidino’.
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-98. ISBN 0-8493-0462-8..
External links
- International Chemical Safety Card 0375
- SIDS Initial Assessment Report for m-Anisidine from the Organisation for Economic Co-operation and Development (OECD)
This article is issued from Wikipedia - version of the 9/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.