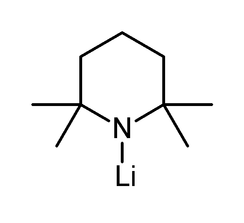
Lithium tetramethylpiperidide
 | |
| Names | |
|---|---|
| IUPAC name
Lithium tetramethylpiperidide | |
| Systematic IUPAC name
1-Lithio-2,2,6,6-tetramethylpiperidine | |
| Identifiers | |
| 38227-87-1 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 21428984 |
| ECHA InfoCard | 100.209.926 |
| PubChem | 11051814 |
| |
| |
| Properties | |
| C9H18LiN | |
| Molar mass | 147.19 g·mol−1 |
| Acidity (pKa) | 37 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lithium tetramethylpiperidide (often abbreviated LiTMP or LTMP) is a chemical compound with the molecular formula C9H18LiN. It is used as a non-nucleophilic base, being comparable to LiHMDS in terms of pKa and steric hindrance.
Synthesis
It is synthesised by the deprotonation of 2,2,6,6-tetramethylpiperidine with n-butyllithium at -78 °C. Recent reports show that this reaction can also be performed 0 °C.[1] The compound is stable in a THF/ethylbenzene solvent mixture and commercially available as such.
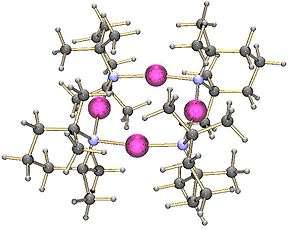
Structure
Like many lithium reagents it has a tendency to aggregate, forming a tetramer in the solid state.[2]
See also
References
- ↑ amide primer H. J. Reich 2002
- ↑ M.F. Lappert, M.J. Slade, A. Singh, J.L. Atwood, R.D. Rogers and R. Shakir (1983). "Structure and reactivity of sterically hindered lithium amides and their diethyl etherates: crystal and molecular structures of [Li{N(SiMe3)2}(OEt2)]2 and tetrakis(2,2,6,6-tetramethylpiperidinatolithium)". Journal of the American Chemical Society. 105 (2): 302–304. doi:10.1021/ja00340a031.
This article is issued from Wikipedia - version of the 9/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.